 |
PDBsum entry 1kp4

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
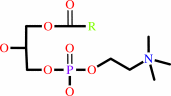 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
277:20059-20069
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
The crystal structure of prokaryotic phospholipase A2.
|
|
Y.Matoba,
Y.Katsube,
M.Sugiyama.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
In this study, the x-ray crystal structures of the calcium-free and
calcium-bound forms of phospholipase A(2) (PLA(2)), produced extracellularly by
Streptomyces violaceoruber, were determined by using the multiple isomorphous
replacement and molecular replacement methods, respectively. The former and
latter structures were refined to an R-factor of 18.8% at a 1.4-A resolution and
an R-factor of 15.0% at a 1.6-A resolution, respectively. The overall structure
of the prokaryotic PLA(2) exhibits a novel folding topology that demonstrates
that it is completely distinct from those of eukaryotic PLA(2)s, which have been
already determined by x-ray and NMR analyses. Furthermore, the coordination
geometry of the calcium(II) ion apparently deviated from that of eukaryotic
PLA(2)s. Regardless of the evolutionary divergence, the catalytic mechanism
including the calcium(II) ion on secreted PLA(2) seems to be conserved between
prokaryotic and eukaryotic cells. Demonstrating that the overall structure
determined by x-ray analysis is almost the same as that determined by NMR
analysis is useful to discuss the catalytic mechanism at the molecular level of
the bacterial PLA(2).

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 5.
Fig. 5. Stereoviews of the catalytic site and surrounding
hydrogen-bonding network. a and b, the calcium-free and
calcium-bound forms of the S. violaceoruver PLA[2],
respectively; c, N. naja atra PLA[2] (7). The hydrogen bonds are
shown by black broken lines. The numbers in b and c show the
distance (Å) between calcium(II) ion and the His64 N  1 (the
His48 N 1 (the
His48 N  1 in N.
naja atra). 1 in N.
naja atra).
|
 |
Figure 6.
Fig. 6. Stereoviews of the calcium-binding site. a and b,
the calcium-free and calcium-bound forms of the S. violaceoruver
PLA[2], respectively; c, N. naja atra PLA[2] (7). A green ball
represents the calcium(II) ion. The hydrogen bonds and
coordination bonds to the calcium (II) ion are shown by black
broken lines.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2002,
277,
20059-20069)
copyright 2002.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.E.Guy,
U.Ståhl,
and
Y.Lindqvist
(2009).
Crystal structure of a class XIB phospholipase A2 (PLA2): rice (oryza sativa) isoform-2 pla2 and an octanoate complex.
|
| |
J Biol Chem,
284,
19371-19379.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Merchant,
R.Heard,
and
C.Monroe
(2009).
Characterization of phospholipase A(2) activity in serum of the American alligator (Alligator mississippiensis).
|
| |
J Exp Zool A Ecol Genet Physiol,
311,
662-666.
|
 |
|
|
|
|
 |
R.J.Dutton,
D.Boyd,
M.Berkmen,
and
J.Beckwith
(2008).
Bacterial species exhibit diversity in their mechanisms and capacity for protein disulfide bond formation.
|
| |
Proc Natl Acad Sci U S A,
105,
11933-11938.
|
 |
|
|
|
|
 |
G.A.Köhler,
A.Brenot,
E.Haas-Stapleton,
N.Agabian,
R.Deva,
and
S.Nigam
(2006).
Phospholipase A2 and phospholipase B activities in fungi.
|
| |
Biochim Biophys Acta,
1761,
1391-1399.
|
 |
|
|
|
|
 |
S.Tomiuk,
and
K.Hofmann
(2003).
Sequence similarity in structurally dissimilar proteins.
|
| |
Curr Biol,
13,
R124-R125.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

