 |
PDBsum entry 2iv2

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2iv2
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.17.98.4
- formate dehydrogenase (acceptor).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
formate + A + H+ = AH2 + CO2
|
 |
 |
 |
 |
 |
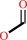 formate
formate
|
+
|
|
+
|
H(+)
|
=
|
AH2
|
+
|
 CO2
CO2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Biol Inorg Chem
11:849-854
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Formate-reduced E. coli formate dehydrogenase H: The reinterpretation of the crystal structure suggests a new reaction mechanism.
|
|
H.C.Raaijmakers,
M.J.Romão.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Re-evaluation of the crystallographic data of the molybdenum-containing E. coli
formate dehydrogenase H (Boyington et al. Science 275:1305-1308, 1997), reported
in two redox states, reveals important structural differences for the
formate-reduced form, with large implications for the reaction mechanism
proposed in that work. We have re-refined the reduced structure with revised
protocols and found substantial rearrangement in some parts of it. The original
model is essentially correct but an important loop close to the molybdenum
active site was mistraced, and, therefore, catalytic relevant residues were
located in wrong positions. In particular selenocysteine-140, a ligand of
molybdenum in the original work, and essential for catalysis, is no longer bound
to the metal after reduction of the enzyme with formate. These results are
incompatible with the originally proposed reaction mechanism. On the basis of
our new interpretation, we have revised and proposed a new reaction mechanism,
which reconciles the new X-ray model with previous biochemical and extended
X-ray absorption fine structure data.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.J.Romão
(2009).
Molybdenum and tungsten enzymes: a crystallographic and mechanistic overview.
|
| |
Dalton Trans,
(),
4053-4068.
|
 |
|
|
|
|
 |
N.M.Cerqueira,
P.J.Gonzalez,
C.D.Brondino,
M.J.Romão,
C.C.Romão,
I.Moura,
and
J.J.Moura
(2009).
The effect of the sixth sulfur ligand in the catalytic mechanism of periplasmic nitrate reductase.
|
| |
J Comput Chem,
30,
2466-2484.
|
 |
|
|
|
|
 |
N.Wagener,
A.J.Pierik,
A.Ibdah,
R.Hille,
and
H.Dobbek
(2009).
The Mo-Se active site of nicotinate dehydrogenase.
|
| |
Proc Natl Acad Sci U S A,
106,
11055-11060.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Groysman,
and
R.H.Holm
(2009).
Biomimetic chemistry of iron, nickel, molybdenum, and tungsten in sulfur-ligated protein sites.
|
| |
Biochemistry,
48,
2310-2320.
|
 |
|
|
|
|
 |
I.Lüke,
G.Butland,
K.Moore,
G.Buchanan,
V.Lyall,
S.A.Fairhurst,
J.F.Greenblatt,
A.Emili,
T.Palmer,
and
F.Sargent
(2008).
Biosynthesis of the respiratory formate dehydrogenases from Escherichia coli: characterization of the FdhE protein.
|
| |
Arch Microbiol,
190,
685-696.
|
 |
|
|
|
|
 |
M.Jormakka,
K.Yokoyama,
T.Yano,
M.Tamakoshi,
S.Akimoto,
T.Shimamura,
P.Curmi,
and
S.Iwata
(2008).
Molecular mechanism of energy conservation in polysulfide respiration.
|
| |
Nat Struct Mol Biol,
15,
730-737.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Sarangi,
S.I.Gorelsky,
L.Basumallick,
H.J.Hwang,
R.C.Pratt,
T.D.Stack,
Y.Lu,
K.O.Hodgson,
B.Hedman,
and
E.I.Solomon
(2008).
Spectroscopic and density functional theory studies of the blue-copper site in M121SeM and C112SeC azurin: Cu-Se versus Cu-S bonding.
|
| |
J Am Chem Soc,
130,
3866-3877.
|
 |
|
|
|
|
 |
S.Najmudin,
P.J.González,
J.Trincão,
C.Coelho,
A.Mukhopadhyay,
N.M.Cerqueira,
C.C.Romão,
I.Moura,
J.J.Moura,
C.D.Brondino,
and
M.J.Romão
(2008).
Periplasmic nitrate reductase revisited: a sulfur atom completes the sixth coordination of the catalytic molybdenum.
|
| |
J Biol Inorg Chem,
13,
737-753.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.W.Ragsdale,
and
E.Pierce
(2008).
Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation.
|
| |
Biochim Biophys Acta,
1784,
1873-1898.
|
 |
|
|
|
|
 |
T.L.Hendrickson
(2007).
Easing selenocysteine into proteins.
|
| |
Nat Struct Mol Biol,
14,
100-101.
|
 |
|
|
|
|
 |
X.Ma,
C.Schulzke,
H.G.Schmidt,
and
M.Noltemeyer
(2007).
Structural, electrochemical and oxygen atom transfer properties of a molybdenum selenoether complex [Mo2O4(OC3H6SeC3H6O)2] and its thioether analogue [Mo2O4(OC3H6SC3H6O)2].
|
| |
Dalton Trans,
(),
1773-1780.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

