 |
PDBsum entry 1xr2

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.1.1.14
- 5-methyltetrahydropteroyltriglutamate--homocysteine S-methyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
5-methyltetrahydropteroyltri-L-glutamate + L-homocysteine = tetrahydropteroyltri-L-glutamate + L-methionine
|
 |
 |
 |
 |
 |
5-methyltetrahydropteroyltri-L-glutamate
Bound ligand (Het Group name = )
matches with 61.54% similarity
|
+
|
L-homocysteine
Bound ligand (Het Group name = )
matches with 45.45% similarity
|
=
|
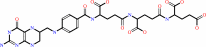 tetrahydropteroyltri-L-glutamate
tetrahydropteroyltri-L-glutamate
|
+
|
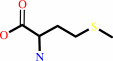 L-methionine
L-methionine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Plos Biol
3:e31-264
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Cobalamin-independent methionine synthase (MetE): a face-to-face double barrel that evolved by gene duplication.
|
|
R.Pejchal,
M.L.Ludwig.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cobalamin-independent methionine synthase (MetE) catalyzes the transfer of a
methyl group from methyltetrahydrofolate to L-homocysteine (Hcy) without using
an intermediate methyl carrier. Although MetE displays no detectable sequence
homology with cobalamin-dependent methionine synthase (MetH), both enzymes
require zinc for activation and binding of Hcy. Crystallographic analyses of
MetE from T. maritima reveal an unusual dual-barrel structure in which the
active site lies between the tops of the two (betaalpha)(8) barrels. The fold of
the N-terminal barrel confirms that it has evolved from the C-terminal
polypeptide by gene duplication; comparisons of the barrels provide an
intriguing example of homologous domain evolution in which binding sites are
obliterated. The C-terminal barrel incorporates the zinc ion that binds and
activates Hcy. The zinc-binding site in MetE is distinguished from the
(Cys)(3)Zn site in the related enzymes, MetH and betaine-homocysteine
methyltransferase, by its position in the barrel and by the metal ligands, which
are histidine, cysteine, glutamate, and cysteine in the resting form of MetE.
Hcy associates at the face of the metal opposite glutamate, which moves away
from the zinc in the binary E.Hcy complex. The folate substrate is not
intimately associated with the N-terminal barrel; instead, elements from both
barrels contribute binding determinants in a binary complex in which the folate
substrate is incorrectly oriented for methyl transfer. Atypical locations of the
Hcy and folate sites in the C-terminal barrel presumably permit direct
interaction of the substrates in a ternary complex. Structures of the binary
substrate complexes imply that rearrangement of folate, perhaps accompanied by
domain rearrangement, must occur before formation of a ternary complex that is
competent for methyl transfer.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.A.Assarehzadegan,
M.Sankian,
F.Jabbari,
M.Tehrani,
R.Falak,
and
A.Varasteh
(2011).
Identification of methionine synthase (Sal k 3), as a novel allergen of Salsola kali pollen.
|
| |
Mol Biol Rep,
38,
65-73.
|
 |
|
|
|
|
 |
A.Roth,
and
R.R.Breaker
(2009).
The structural and functional diversity of metabolite-binding riboswitches.
|
| |
Annu Rev Biochem,
78,
305-334.
|
 |
|
|
|
|
 |
E.R.Hondorp,
and
R.G.Matthews
(2009).
Oxidation of cysteine 645 of cobalamin-independent methionine synthase causes a methionine limitation in Escherichia coli.
|
| |
J Bacteriol,
191,
3407-3410.
|
 |
|
|
|
|
 |
Y.Zhang,
D.A.Rodionov,
M.S.Gelfand,
and
V.N.Gladyshev
(2009).
Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization.
|
| |
BMC Genomics,
10,
78.
|
 |
|
|
|
|
 |
A.M.Krishnakumar,
D.Sliwa,
J.A.Endrizzi,
E.S.Boyd,
S.A.Ensign,
and
J.W.Peters
(2008).
Getting a handle on the role of coenzyme M in alkene metabolism.
|
| |
Microbiol Mol Biol Rev,
72,
445-456.
|
 |
|
|
|
|
 |
M.J.Schneider,
M.Ulland,
and
R.D.Sloboda
(2008).
A protein methylation pathway in Chlamydomonas flagella is active during flagellar resorption.
|
| |
Mol Biol Cell,
19,
4319-4327.
|
 |
|
|
|
|
 |
M.Koutmos,
R.Pejchal,
T.M.Bomer,
R.G.Matthews,
J.L.Smith,
and
M.L.Ludwig
(2008).
Metal active site elasticity linked to activation of homocysteine in methionine synthases.
|
| |
Proc Natl Acad Sci U S A,
105,
3286-3291.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.Morris,
L.J.Marinelli,
D.Jacobs-Sera,
R.W.Hendrix,
and
G.F.Hatfull
(2008).
Genomic characterization of mycobacteriophage Giles: evidence for phage acquisition of host DNA by illegitimate recombination.
|
| |
J Bacteriol,
190,
2172-2182.
|
 |
|
|
|
|
 |
S.W.Ragsdale
(2008).
Catalysis of methyl group transfers involving tetrahydrofolate and B(12).
|
| |
Vitam Horm,
79,
293-324.
|
 |
|
|
|
|
 |
B.Li,
and
W.A.van der Donk
(2007).
Identification of essential catalytic residues of the cyclase NisC involved in the biosynthesis of nisin.
|
| |
J Biol Chem,
282,
21169-21175.
|
 |
|
|
|
|
 |
F.Rébeillé,
S.Ravanel,
A.Marquet,
R.R.Mendel,
M.E.Webb,
A.G.Smith,
and
M.J.Warren
(2007).
Roles of vitamins B5, B8, B9, B12 and molybdenum cofactor at cellular and organismal levels.
|
| |
Nat Prod Rep,
24,
949-962.
|
 |
|
|
|
|
 |
H.S.Suliman,
D.R.Appling,
and
J.D.Robertus
(2007).
The gene for cobalamin-independent methionine synthase is essential in Candida albicans: a potential antifungal target.
|
| |
Arch Biochem Biophys,
467,
218-226.
|
 |
|
|
|
|
 |
J.Penner-Hahn
(2007).
Zinc-promoted alkyl transfer: a new role for zinc.
|
| |
Curr Opin Chem Biol,
11,
166-171.
|
 |
|
|
|
|
 |
M.Paul,
G.C.Patton,
and
W.A.van der Donk
(2007).
Mutants of the zinc ligands of lacticin 481 synthetase retain dehydration activity but have impaired cyclization activity.
|
| |
Biochemistry,
46,
6268-6276.
|
 |
|
|
|
|
 |
B.Li,
J.P.Yu,
J.S.Brunzelle,
G.N.Moll,
W.A.van der Donk,
and
S.K.Nair
(2006).
Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis.
|
| |
Science,
311,
1464-1467.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Sudarsan,
M.C.Hammond,
K.F.Block,
R.Welz,
J.E.Barrick,
A.Roth,
and
R.R.Breaker
(2006).
Tandem riboswitch architectures exhibit complex gene control functions.
|
| |
Science,
314,
300-304.
|
 |
|
|
|
|
 |
R.E.Taurog,
H.Jakubowski,
and
R.G.Matthews
(2006).
Synergistic, random sequential binding of substrates in cobalamin-independent methionine synthase.
|
| |
Biochemistry,
45,
5083-5091.
|
 |
|
|
|
|
 |
R.E.Taurog,
and
R.G.Matthews
(2006).
Activation of methyltetrahydrofolate by cobalamin-independent methionine synthase.
|
| |
Biochemistry,
45,
5092-5102.
|
 |
|
|
|
|
 |
T.M.Fu,
X.Y.Zhang,
L.F.Li,
Y.H.Liang,
and
X.D.Su
(2006).
Preparation, crystallization and preliminary X-ray analysis of the methionine synthase (MetE) from Streptococcus mutans.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
984-985.
|
 |
|
|
|
|
 |
L.Huang,
D.Y.Li,
S.X.Wang,
S.M.Zhang,
J.H.Chen,
and
X.F.Wu
(2005).
Cloning and identification of methionine synthase gene from Pichia pastoris.
|
| |
Acta Biochim Biophys Sin (Shanghai),
37,
371-378.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

