
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
A short peptide insertion crucial for angiostatic activity of human tryptophanyl-tRNA synthetase
|
|
Structure:
|
 |
Tryptophanyl-tRNA synthetase. Chain: a, b. Fragment: residues 35-424. Synonym: tryptophan--tRNA ligase, trprs, ifp53, hwrs, mini trprs. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: trps. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008.
|
|
Biol. unit:
|
 |
Dimer (from
)
|
|
Resolution:
|
 |
|
2.31Å
|
R-factor:
|
0.241
|
R-free:
|
0.292
|
|
|
Authors:
|
 |
Y.Kise,T.Sengoku,R.Ishii,S.Yokoyama,S.G.Park,S.W.Lee,S.Kim,O.Nureki, Riken Structural Genomics/proteomics Initiative (Rsgi)
|
Key ref:

|
 |
Y.Kise
et al.
(2004).
A short peptide insertion crucial for angiostatic activity of human tryptophanyl-tRNA synthetase.
Nat Struct Mol Biol,
11,
149-156.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
12-Sep-03
|
Release date:
|
03-Feb-04
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.6.1.1.2
- tryptophan--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Trp) + L-tryptophan + ATP = L-tryptophyl-tRNA(Trp) + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
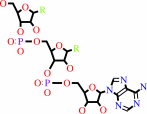 tRNA(Trp)
tRNA(Trp)
|
+
|
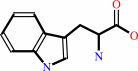 L-tryptophan
L-tryptophan
|
+
|
 ATP
ATP
|
=
|
L-tryptophyl-tRNA(Trp)
|
+
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nat Struct Mol Biol
11:149-156
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
A short peptide insertion crucial for angiostatic activity of human tryptophanyl-tRNA synthetase.
|
|
Y.Kise,
S.W.Lee,
S.G.Park,
S.Fukai,
T.Sengoku,
R.Ishii,
S.Yokoyama,
S.Kim,
O.Nureki.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Human tryptophanyl-tRNA synthetase (TrpRS) is secreted into the extracellular
region of vascular endothelial cells. The splice variant form (mini TrpRS)
functions in vascular endothelial cell apoptosis as an angiostatic cytokine. In
contrast, the closely related human tyrosyl-tRNA synthetase (TyrRS) functions as
an angiogenic cytokine in its truncated form (mini TyrRS). Here, we determined
the crystal structure of human mini TrpRS at a resolution of 2.3 A and compared
the structure with those of prokaryotic TrpRS and human mini TyrRS. Deletion of
the tRNA anticodon-binding (TAB) domain insertion, consisting of eight residues
in the human TrpRS, abolished the enzyme's apoptotic activity for endothelial
cells, whereas its translational catalysis and cell-binding activities remained
unchanged. Thus, we have identified the inserted peptide motif that activates
the angiostatic signaling.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. Structure of the human mini TrpRS structure. (a)
Stereo view of the N-terminal extension domain (residues 82
-154), the Trp-tRNA^Trp synthesizing Rossmann fold domain
(residues 155 -246 and 293 -347), the CP1 insertion domain
(residues 247 -292) and the TAB domain (residues 348 -471) are
shown in blue, green, red and yellow, respectively. The
secondary structures are numerically labeled. (b) Overall
structure of the mini TrpRS dimer. One monomer is colored as in
a, whereas the other is colored differently for the respective
domains.
|
 |
Figure 2.
Figure 2. Structural comparison. (a) Human mini TrpRS. (b) B.
stearothermophilus TrpRS. (c) human mini TyrRS. Color coding is
as in Figure 1a. The unique structural features in each protein
are circled. The TrpRS-specific structures are indicated as M1,
M2, M3 and M4. The YGY sequence of mini TrpRS and the ELR motif
of mini TyrRS are indicated.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nat Struct Mol Biol
(2004,
11,
149-156)
copyright 2004.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.Guo,
X.L.Yang,
and
P.Schimmel
(2010).
New functions of aminoacyl-tRNA synthetases beyond translation.
|
| |
Nat Rev Mol Cell Biol,
11,
668-674.
|
 |
|
|
|
|
 |
M.Zhou,
X.Dong,
N.Shen,
C.Zhong,
and
J.Ding
(2010).
Crystal structures of Saccharomyces cerevisiae tryptophanyl-tRNA synthetase: new insights into the mechanism of tryptophan activation and implications for anti-fungal drug design.
|
| |
Nucleic Acids Res,
38,
3399-3413.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Q.Zhou,
M.Kapoor,
M.Guo,
R.Belani,
X.Xu,
W.B.Kiosses,
M.Hanan,
C.Park,
E.Armour,
M.H.Do,
L.A.Nangle,
P.Schimmel,
and
X.L.Yang
(2010).
Orthogonal use of a human tRNA synthetase active site to achieve multifunctionality.
|
| |
Nat Struct Mol Biol,
17,
57-61.
|
 |
|
|
|
|
 |
R.Zeng,
Y.C.Chen,
Z.Zeng,
W.Q.Liu,
X.X.Liu,
R.Liu,
O.Qiang,
and
X.Li
(2010).
Different angiogenesis effect of mini-TyrRS/mini-TrpRS by systemic administration of modified siRNAs in rats with acute myocardial infarction.
|
| |
Heart Vessels,
25,
324-332.
|
 |
|
|
|
|
 |
W.Herzog,
K.Müller,
J.Huisken,
and
D.Y.Stainier
(2009).
Genetic evidence for a noncanonical function of seryl-tRNA synthetase in vascular development.
|
| |
Circ Res,
104,
1260-1266.
|
 |
|
|
|
|
 |
C.D.Hausmann,
and
M.Ibba
(2008).
Aminoacyl-tRNA synthetase complexes: molecular multitasking revealed.
|
| |
FEMS Microbiol Rev,
32,
705-721.
|
 |
|
|
|
|
 |
J.Jia,
A.Arif,
P.S.Ray,
and
P.L.Fox
(2008).
WHEP domains direct noncanonical function of glutamyl-Prolyl tRNA synthetase in translational control of gene expression.
|
| |
Mol Cell,
29,
679-690.
|
 |
|
|
|
|
 |
M.Kapoor,
Q.Zhou,
F.Otero,
C.A.Myers,
A.Bates,
R.Belani,
J.Liu,
J.K.Luo,
E.Tzima,
D.E.Zhang,
X.L.Yang,
and
P.Schimmel
(2008).
Evidence for Annexin II-S100A10 Complex and Plasmin in Mobilization of Cytokine Activity of Human TrpRS.
|
| |
J Biol Chem,
283,
2070-2077.
|
 |
|
|
|
|
 |
N.Shen,
M.Zhou,
B.Yang,
Y.Yu,
X.Dong,
and
J.Ding
(2008).
Catalytic mechanism of the tryptophan activation reaction revealed by crystal structures of human tryptophanyl-tRNA synthetase in different enzymatic states.
|
| |
Nucleic Acids Res,
36,
1288-1299.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.Zhou,
J.Teruya-Feldstein,
P.Lu,
M.Fleisher,
A.Olshen,
and
R.L.Comenzo
(2008).
Calreticulin expression in the clonal plasma cells of patients with systemic light-chain (AL-) amyloidosis is associated with response to high-dose melphalan.
|
| |
Blood,
111,
549-557.
|
 |
|
|
|
|
 |
R.Zeng,
Y.C.Chen,
Z.Zeng,
R.Liu,
O.Qiang,
X.F.Jiang,
X.X.Liu,
X.Li,
and
H.Y.Wang
(2008).
Small interfering RNA knockdown of mini-TyrRS and mini-TrpRS effects angiogenesis in human umbilical vein endothelial cells in hypoxic culture.
|
| |
Cytotechnology,
56,
219-231.
|
 |
|
|
|
|
 |
S.G.Park,
P.Schimmel,
and
S.Kim
(2008).
Aminoacyl tRNA synthetases and their connections to disease.
|
| |
Proc Natl Acad Sci U S A,
105,
11043-11049.
|
 |
|
|
|
|
 |
S.H.Shin,
H.S.Kim,
S.H.Jung,
H.D.Xu,
Y.B.Jeong,
and
Y.J.Chung
(2008).
Implication of leucyl-tRNA synthetase 1 (LARS1) over-expression in growth and migration of lung cancer cells detected by siRNA targeted knock-down analysis.
|
| |
Exp Mol Med,
40,
229-236.
|
 |
|
|
|
|
 |
X.L.Yang,
M.Guo,
M.Kapoor,
K.L.Ewalt,
F.J.Otero,
R.J.Skene,
D.E.McRee,
and
P.Schimmel
(2007).
Functional and crystal structure analysis of active site adaptations of a potent anti-angiogenic human tRNA synthetase.
|
| |
Structure,
15,
793-805.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.Tzima,
and
P.Schimmel
(2006).
Inhibition of tumor angiogenesis by a natural fragment of a tRNA synthetase.
|
| |
Trends Biochem Sci,
31,
7.
|
 |
|
|
|
|
 |
N.Shen,
L.Guo,
B.Yang,
Y.Jin,
and
J.Ding
(2006).
Structure of human tryptophanyl-tRNA synthetase in complex with tRNATrp reveals the molecular basis of tRNA recognition and specificity.
|
| |
Nucleic Acids Res,
34,
3246-3258.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.N.Re,
and
J.L.Cook
(2006).
An intracrine view of angiogenesis.
|
| |
Bioessays,
28,
943-953.
|
 |
|
|
|
|
 |
X.L.Yang,
F.J.Otero,
K.L.Ewalt,
J.Liu,
M.A.Swairjo,
C.Köhrer,
U.L.RajBhandary,
R.J.Skene,
D.E.McRee,
and
P.Schimmel
(2006).
Two conformations of a crystalline human tRNA synthetase-tRNA complex: implications for protein synthesis.
|
| |
EMBO J,
25,
2919-2929.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.Tzima,
J.S.Reader,
M.Irani-Tehrani,
K.L.Ewalt,
M.A.Schwartz,
and
P.Schimmel
(2005).
VE-cadherin links tRNA synthetase cytokine to anti-angiogenic function.
|
| |
J Biol Chem,
280,
2405-2408.
|
 |
|
|
|
|
 |
S.G.Park,
H.J.Kim,
Y.H.Min,
E.C.Choi,
Y.K.Shin,
B.J.Park,
S.W.Lee,
and
S.Kim
(2005).
Human lysyl-tRNA synthetase is secreted to trigger proinflammatory response.
|
| |
Proc Natl Acad Sci U S A,
102,
6356-6361.
|
 |
|
|
|
|
 |
S.G.Park,
K.L.Ewalt,
and
S.Kim
(2005).
Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers.
|
| |
Trends Biochem Sci,
30,
569-574.
|
 |
|
|
|
|
 |
J.Jia,
X.L.Chen,
L.T.Guo,
Y.D.Yu,
J.P.Ding,
and
Y.X.Jin
(2004).
Residues Lys-149 and Glu-153 switch the aminoacylation of tRNA(Trp) in Bacillus subtilis.
|
| |
J Biol Chem,
279,
41960-41965.
|
 |
|
|
|
|
 |
Y.G.Zheng,
H.Wei,
C.Ling,
F.Martin,
G.Eriani,
and
E.D.Wang
(2004).
Two distinct domains of the beta subunit of Aquifex aeolicus leucyl-tRNA synthetase are involved in tRNA binding as revealed by a three-hybrid selection.
|
| |
Nucleic Acids Res,
32,
3294-3303.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

