
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Zn-binding domain of the t347g mutant of human aminoacylase-i
|
|
Structure:
|
 |
Aminoacylase-1. Chain: a, c. Fragment: zn-binding domain (residues 1-198). Synonym: n-acyl-l-amino-acid amidohydrolase, acy-1. Engineered: yes. Aminoacylase-1. Chain: b, d. Fragment: residues 321-408. Synonym: n-acyl-l-amino-acid amidohydrolase, acy-1.
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: acy1. Expressed in: spodoptera frugiperda. Expression_system_taxid: 7108.
|
|
Biol. unit:
|
 |
Tetramer (from
)
|
|
Resolution:
|
 |
|
1.40Å
|
R-factor:
|
0.133
|
R-free:
|
0.172
|
|
|
Authors:
|
 |
H.A.Lindner,V.V.Lunin,A.Alary,R.Hecker,M.Cygler,R.Menard
|
Key ref:

|
 |
H.A.Lindner
et al.
(2003).
Essential roles of zinc ligation and enzyme dimerization for catalysis in the aminoacylase-1/M20 family.
J Biol Chem,
278,
44496-44504.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
19-Aug-03
|
Release date:
|
20-Jan-04
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D:
E.C.3.5.1.14
- N-acyl-aliphatic-L-amino acid amidohydrolase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
an N-acyl-L-amino acid + H2O = an L-alpha-amino acid + a carboxylate
|
|
2.
|
an N-acetyl-L-cysteine-S-conjugate + H2O = an S-substituted L-cysteine + acetate
|
|
 |
 |
 |
 |
 |
N-acyl-L-amino acid
Bound ligand (Het Group name = )
matches with 55.56% similarity
|
+
|
H2O
|
=
|
L-alpha-amino acid
|
+
|
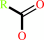 carboxylate
carboxylate
|
|
 |
 |
 |
 |
 |
N-acetyl-L-cysteine-S-conjugate
|
+
|
H2O
|
=
|
S-substituted L-cysteine
Bound ligand (Het Group name = )
matches with 80.00% similarity
|
+
|
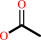 acetate
acetate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
278:44496-44504
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Essential roles of zinc ligation and enzyme dimerization for catalysis in the aminoacylase-1/M20 family.
|
|
H.A.Lindner,
V.V.Lunin,
A.Alary,
R.Hecker,
M.Cygler,
R.Ménard.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Members of the aminoacylase-1 (Acy1)/M20 family of aminoacylases and
exopeptidases exist as either monomers or homodimers. They contain a
zinc-binding domain and a second domain mediating dimerization in the latter
case. The roles that both domains play in catalysis have been investigated for
human Acy1 (hAcy1) by x-ray crystallography and by site-directed mutagenesis.
Structure comparison of the dinuclear zinc center in a mutant of hAcy1 reported
here with dizinc centers in related enzymes points to a difference in zinc
ligation in the Acy1/M20 family. Mutational analysis supports catalytic roles of
zinc ions, a vicinal glutamate, and a histidine from the dimerization domain. By
complementing different active site mutants of hAcy1, we show that catalysis
occurs at the dimer interface. Reinterpretation of the structure of a monomeric
homolog, peptidase V, reveals that a domain insertion mimics dimerization. We
conclude that monomeric and dimeric Acy1/M20 family members share a unique
active site architecture involving both enzyme domains. The study may provide
means to improve homologous carboxypeptidase G2 toward application in
antibody-directed enzyme prodrug therapy.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIG. 1. Ribbon diagram of the zinc-binding domain in the
T347G mutant of hAcy1. Glycine was modeled in place of a
putative L-norleucine ligand molecule and is shown in a
ball-and-stick representation. Zinc ions are represented as gray
spheres.
|
 |
Figure 3.
FIG. 3. Structures of the small domains of enzymes from the
Acy1/M20 family. A, topology diagram for the lid domain in L.
delbrueckii PepV and the dimerization domains from both monomers
in Pseudomonas sp. CPG2. Subdomains 1 (gray) and 2 (white) of
PepV show apparent similarity. However, strands 8 and 12 are
only found in subdomain 1, and strands 3 and 7 are only found in
subdomain 2. The  -sheet composed of the
latter two strands is also present in the dimerization domain of
CPG2. B, backbone trace superposition of subdomains 1 and 2 in
the lid domain of PepV (blue) and the two associated
dimerization domains in CPG2 (red and green). Known active site
residues in PepV are shown in a stick representation, from left
to right, Arg350, Asn217 (both carboxyl-terminal docking), and
His269 (transition state stabilization). Corresponding residues
from CPG2 are also shown. The enlargement above additionally
shows the corresponding residues in PepT. Arg288 from CPG2 (red)
and Arg280 from PepT (yellow) reside in the monomer, which
superimposes with subdomain 1 of PepV. Asn275 and His229 from
CPG2 (green) and His223 in PepT (purple) are recruited from the
opposite monomer which superimposes with subdomain 2 of PepV. In
the structure of CPG2, the side chain of His229 shows a -sheet composed of the
latter two strands is also present in the dimerization domain of
CPG2. B, backbone trace superposition of subdomains 1 and 2 in
the lid domain of PepV (blue) and the two associated
dimerization domains in CPG2 (red and green). Known active site
residues in PepV are shown in a stick representation, from left
to right, Arg350, Asn217 (both carboxyl-terminal docking), and
His269 (transition state stabilization). Corresponding residues
from CPG2 are also shown. The enlargement above additionally
shows the corresponding residues in PepT. Arg288 from CPG2 (red)
and Arg280 from PepT (yellow) reside in the monomer, which
superimposes with subdomain 1 of PepV. Asn275 and His229 from
CPG2 (green) and His223 in PepT (purple) are recruited from the
opposite monomer which superimposes with subdomain 2 of PepV. In
the structure of CPG2, the side chain of His229 shows a  [1]
rotation by about 90° relative to the other two structures
and coordinates an additional interdimeric zinc ion in the
protein crystal (not shown). C, multiple sequence alignment of
the small domains in the PepV enzymes from L. delbrueckii
(PEPV_LACDL) and Lactococcus lactis subsp. cremoris MG1363
(PEPV_LACLC) and from CPG2 (CBPG_PSES6), PepT (PEPT_SALTY), and
hAcy1 (ACY1_HUMAN). Subdomain 1 and 2 in the lid domain of PepV
are abbreviated sd1 and sd2, respectively. The alignment was
assembled using an available alignment of the two PepV enzymes
(15) and structure-based alignments of CPG2 to sd1 in L.
Delbrueckii (29) and CPG2 to PepT (24). The sequences of the
dimerization domain in hAcy1 and CPG2 were aligned manually.
Strands (s) and helices (h), as identified in the crystal
structures of PepV, CPG2, and PepT, are printed in red and blue,
respectively. Their numbering in sd1 and sd2 of L. delbrueckii
PepV is indicated in the corresponding colors above the aligned
sequences. Residues that interact with the bound transition
state analog Asp [1]
rotation by about 90° relative to the other two structures
and coordinates an additional interdimeric zinc ion in the
protein crystal (not shown). C, multiple sequence alignment of
the small domains in the PepV enzymes from L. delbrueckii
(PEPV_LACDL) and Lactococcus lactis subsp. cremoris MG1363
(PEPV_LACLC) and from CPG2 (CBPG_PSES6), PepT (PEPT_SALTY), and
hAcy1 (ACY1_HUMAN). Subdomain 1 and 2 in the lid domain of PepV
are abbreviated sd1 and sd2, respectively. The alignment was
assembled using an available alignment of the two PepV enzymes
(15) and structure-based alignments of CPG2 to sd1 in L.
Delbrueckii (29) and CPG2 to PepT (24). The sequences of the
dimerization domain in hAcy1 and CPG2 were aligned manually.
Strands (s) and helices (h), as identified in the crystal
structures of PepV, CPG2, and PepT, are printed in red and blue,
respectively. Their numbering in sd1 and sd2 of L. delbrueckii
PepV is indicated in the corresponding colors above the aligned
sequences. Residues that interact with the bound transition
state analog Asp  [PO[2]CH[2]]AlaOH in the
PepV structure are in yellow boxes. Greek letters indicate the
sites of rearrangement generated by the insertion of sd2 in the
sequence of sd1 and their sequel. [PO[2]CH[2]]AlaOH in the
PepV structure are in yellow boxes. Greek letters indicate the
sites of rearrangement generated by the insertion of sd2 in the
sequence of sd1 and their sequel.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2003,
278,
44496-44504)
copyright 2003.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Andrady,
S.K.Sharma,
and
K.A.Chester
(2011).
Antibody-enzyme fusion proteins for cancer therapy.
|
| |
Immunotherapy,
3,
193-211.
|
 |
|
|
|
|
 |
J.M.Hsieh,
K.Tsirulnikov,
M.R.Sawaya,
N.Magilnick,
N.Abuladze,
I.Kurtz,
J.Abramson,
and
A.Pushkin
(2010).
Structures of aminoacylase 3 in complex with acetylated substrates.
|
| |
Proc Natl Acad Sci U S A,
107,
17962-17967.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.S.Girish,
and
B.Gopal
(2010).
Crystal structure of Staphylococcus aureus metallopeptidase (Sapep) reveals large domain motions between the manganese-bound and apo-states.
|
| |
J Biol Chem,
285,
29406-29415.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.Y.Chang,
Y.C.Hsieh,
T.Y.Wang,
C.J.Chen,
and
T.K.Wu
(2009).
Purification, crystallization and preliminary X-ray analysis of an aminoacylhistidine dipeptidase (PepD) from Vibrio alginolyticus.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
216-218.
|
 |
|
|
|
|
 |
K.Tsirulnikov,
N.Abuladze,
D.Newman,
S.Ryazantsev,
T.Wolak,
N.Magilnick,
M.C.Koag,
I.Kurtz,
and
A.Pushkin
(2009).
Mouse aminoacylase 3: a metalloenzyme activated by cobalt and nickel.
|
| |
Biochim Biophys Acta,
1794,
1049-1057.
|
 |
|
|
|
|
 |
Y.Zhong,
J.Onuki,
T.Yamasaki,
O.Ogawa,
S.Akatsuka,
and
S.Toyokuni
(2009).
Genome-wide analysis identifies a tumor suppressor role for aminoacylase 1 in iron-induced rat renal cell carcinoma.
|
| |
Carcinogenesis,
30,
158-164.
|
 |
|
|
|
|
 |
K.Tanimoto,
N.Higashi,
M.Nishioka,
K.Ishikawa,
and
M.Taya
(2008).
Characterization of thermostable aminoacylase from hyperthermophilic archaeon Pyrococcus horikoshii.
|
| |
FEBS J,
275,
1140-1149.
|
 |
|
|
|
|
 |
D.Hedley,
L.Ogilvie,
and
C.Springer
(2007).
Carboxypeptidase-G2-based gene-directed enzyme-prodrug therapy: a new weapon in the GDEPT armoury.
|
| |
Nat Rev Cancer,
7,
870-879.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

