
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|

 223 a.a.
223 a.a.
|
 |

|

|

|

|

|

|

|
 1113 a.a.
1113 a.a.
|
 |

|

|

|

|

|

|

|
 1175 a.a.
1175 a.a.
|
 |

|

|

|

|

|

|

|
 98 a.a.
98 a.a.
|
 |

|

|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of thermus aquaticus core RNA polymerase-includes complete structure with side-chains (except for disordered regions)- further refined from original deposition-contains additional sequence information
|
|
Structure:
|
 |
DNA-directed RNA polymerase subunit alpha. Chain: a, b. Synonym: rnap subunit alpha,RNA polymerase subunit alpha, transcriptase subunit alpha. DNA-directed RNA polymerase subunit beta. Chain: c. Synonym: rnap subunit beta,RNA polymerase subunit beta,transcriptase subunit beta. DNA-directed RNA polymerase subunit beta'.
|
|
Source:
|
 |
Thermus aquaticus. Organism_taxid: 271. Organism_taxid: 271
|
|
Biol. unit:
|
 |
Pentamer (from
)
|
|
Resolution:
|
 |
|
3.30Å
|
R-factor:
|
0.300
|
R-free:
|
0.360
|
|
|
Authors:
|
 |
L.Minakhin,S.Bhagat,A.Brunning,E.A.Campbell,S.A.Darst,R.H.Ebright, K.Severinov
|
Key ref:

|
 |
L.Minakhin
et al.
(2001).
Bacterial RNA polymerase subunit omega and eukaryotic RNA polymerase subunit RPB6 are sequence, structural, and functional homologs and promote RNA polymerase assembly.
Proc Natl Acad Sci U S A,
98,
892-897.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
18-Dec-00
|
Release date:
|
07-Feb-01
|
|
|
Supersedes:
|

|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9KWU8
(RPOA_THEAQ) -
DNA-directed RNA polymerase subunit alpha from Thermus aquaticus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
314 a.a.
223 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
Q9KWU7
(RPOB_THEAQ) -
DNA-directed RNA polymerase subunit beta from Thermus aquaticus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
1119 a.a.
1113 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D, E:
E.C.2.7.7.6
- DNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
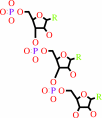 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
98:892-897
(2001)
|
|
PubMed id:
|
|
|
|

|
| |
|
Bacterial RNA polymerase subunit omega and eukaryotic RNA polymerase subunit RPB6 are sequence, structural, and functional homologs and promote RNA polymerase assembly.
|
|
L.Minakhin,
S.Bhagat,
A.Brunning,
E.A.Campbell,
S.A.Darst,
R.H.Ebright,
K.Severinov.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Bacterial DNA-dependent RNA polymerase (RNAP) has subunit composition
beta'betaalpha(I)alpha(II)omega. The role of omega has been unclear. We show
that omega is homologous in sequence and structure to RPB6, an essential subunit
shared in eukaryotic RNAP I, II, and III. In Escherichia coli, overproduction of
omega suppresses the assembly defect caused by substitution of residue 1362 of
the largest subunit of RNAP, beta'. In yeast, overproduction of RPB6 suppresses
the assembly defect caused by the equivalent substitution in the largest subunit
of RNAP II, RPB1. High-resolution structural analysis of the omega-beta'
interface in bacterial RNAP, and comparison with the RPB6-RPB1 interface in
yeast RNAP II, confirms the structural relationship and suggests a
"latching" mechanism for the role of omega and RPB6 in promoting RNAP
assembly.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig. 2. Structure determination. (a) Stereo view of a
portion of the 2|F[o]|  |F[c]|
electron density map (3.2 Å, 1 |F[c]|
electron density map (3.2 Å, 1  , shown in
blue) calculated from the T. aquaticus core RNAP structure,
showing a region corresponding to the , shown in
blue) calculated from the T. aquaticus core RNAP structure,
showing a region corresponding to the  subunit
(including the N-terminal part of CR1 subunit
(including the N-terminal part of CR1  -helix; at
center, oriented horizontally) and nearby parts of -helix; at
center, oriented horizontally) and nearby parts of  and and  '. Atoms of '. Atoms of
 are
colored by atom type (C, yellow; O, red; N, blue; S, green).
Atoms of are
colored by atom type (C, yellow; O, red; N, blue; S, green).
Atoms of  ' and ' and  are colored
pink and light blue, respectively. The SeMet difference Fourier
peak (3 are colored
pink and light blue, respectively. The SeMet difference Fourier
peak (3  ) that
corresponds to Met12 of ) that
corresponds to Met12 of  is shown
in magenta. Selected residues of is shown
in magenta. Selected residues of  are
labeled. The figure was generated by using the program O (40).
(b) Structure of the are
labeled. The figure was generated by using the program O (40).
(b) Structure of the  subunit in
T. aquaticus RNAP core enzyme. A ribbon representation of T.
aquaticus subunit in
T. aquaticus RNAP core enzyme. A ribbon representation of T.
aquaticus  residues
(residues 2-96) is shown. Residues of residues
(residues 2-96) is shown. Residues of  not
included in the sequence alignment in Fig. 1 are illustrated in
white; conserved regions CR1-CR3 are in yellow; nonconserved
regions are in cyan. S1 is part of intersubunit not
included in the sequence alignment in Fig. 1 are illustrated in
white; conserved regions CR1-CR3 are in yellow; nonconserved
regions are in cyan. S1 is part of intersubunit  -sheet
(two-strand antiparallel -sheet
(two-strand antiparallel  -sheet with
residues 1483-1487 of the C-terminal tail of -sheet with
residues 1483-1487 of the C-terminal tail of  '). ').
|
 |
Figure 3.
Fig. 3. Bacterial  and
eukaryotic RPB6 are structural homologs. (a) Structure of and
eukaryotic RPB6 are structural homologs. (a) Structure of  and the and the
 - -  ' interface
in T. aquaticus RNAP core enzyme. Residues of ' interface
in T. aquaticus RNAP core enzyme. Residues of  included
in the sequence alignment in Fig. 1 are illustrated in a ribbon
representation (residues 9-81); conserved regions CR1-CR3 are in
yellow; nonconserved regions are in cyan. Residues of included
in the sequence alignment in Fig. 1 are illustrated in a ribbon
representation (residues 9-81); conserved regions CR1-CR3 are in
yellow; nonconserved regions are in cyan. Residues of  ' conserved
regions D and G are in pink; residues of the ' conserved
regions D and G are in pink; residues of the  ' C-terminal
tail are in red, with the residue corresponding to the residue
substituted in the E. coli rpoC^tsx mutant (panel f and Fig. 4a)
indicated in green. (b) Location of ' C-terminal
tail are in red, with the residue corresponding to the residue
substituted in the E. coli rpoC^tsx mutant (panel f and Fig. 4a)
indicated in green. (b) Location of  within T.
aquaticus RNAP core enzyme. The structure of T. aquaticus RNAP
core enzyme is illustrated in a C within T.
aquaticus RNAP core enzyme. The structure of T. aquaticus RNAP
core enzyme is illustrated in a C  representation.
Conserved regions CR1-CR3 of representation.
Conserved regions CR1-CR3 of  are in
yellow; nonconserved regions of are in
yellow; nonconserved regions of  are in
blue; are in
blue;  ' is in
pink, with the ' is in
pink, with the  ' C-terminal
tail in red; ' C-terminal
tail in red;  is in cyan, is in cyan,
 I and I and  II are in
green; the active-center Mg2+ is in magenta. (c) Structure of
RPB6 and the RPB6-RPB1 interface in yeast RNAP II (atomic
coordinates from ref. 2, PDB accession code 1EN0, with
reassignment of 79 C-terminal residues of RPB1 based on refined
atomic coordinates) (P. Cramer, D. Bushnell, and R. Kornberg,
personal communication). Residues of RPB6 included in the
sequence alignment in Fig. 1 are illustrated in a ribbon
representation (residues 80-138); conserved regions CR1-CR3 are
in yellow; nonconserved regions are in cyan. Residues of RPB1
conserved regions D and G are in pink; residues of the RPB1
C-terminal tail are in red, with the residue substituted in
RPB1-1 (panel f and Fig. 4b) indicated in green. (Residues of
the C-terminal tail following residue Ile^1445 are not defined
in the available structure.) Residue numbers in structural
elements of RPB6 and RPB1 were inferred by reference to residue
numbers in structurally equivalent elements of II are in
green; the active-center Mg2+ is in magenta. (c) Structure of
RPB6 and the RPB6-RPB1 interface in yeast RNAP II (atomic
coordinates from ref. 2, PDB accession code 1EN0, with
reassignment of 79 C-terminal residues of RPB1 based on refined
atomic coordinates) (P. Cramer, D. Bushnell, and R. Kornberg,
personal communication). Residues of RPB6 included in the
sequence alignment in Fig. 1 are illustrated in a ribbon
representation (residues 80-138); conserved regions CR1-CR3 are
in yellow; nonconserved regions are in cyan. Residues of RPB1
conserved regions D and G are in pink; residues of the RPB1
C-terminal tail are in red, with the residue substituted in
RPB1-1 (panel f and Fig. 4b) indicated in green. (Residues of
the C-terminal tail following residue Ile^1445 are not defined
in the available structure.) Residue numbers in structural
elements of RPB6 and RPB1 were inferred by reference to residue
numbers in structurally equivalent elements of  and and  ' (a) and to
sequence alignments (Fig. 1; also panel f herein), and are
expected to be correct within ' (a) and to
sequence alignments (Fig. 1; also panel f herein), and are
expected to be correct within  1 residue. (d)
Location of RPB6 within yeast RNAP II (atomic coordinates as in
c). The structure of yeast RNAP II is illustrated in a C 1 residue. (d)
Location of RPB6 within yeast RNAP II (atomic coordinates as in
c). The structure of yeast RNAP II is illustrated in a C  representation.
Conserved regions CR1-CR3 of RPB6 are in yellow; nonconserved
regions of RPB6 are in blue; RPB1 is in pink, with the RPB1
C-terminal tail in red; RPB2 is in cyan, RPB3 and RPB11 are in
green; subunits of RNAP II without counterparts in bacterial
RNAP (RPB5, RPB8, RPB9, RPB10, and RPB12) are in gray; the
active-center Mg2+ is in magenta. (e) Structural alignment of representation.
Conserved regions CR1-CR3 of RPB6 are in yellow; nonconserved
regions of RPB6 are in blue; RPB1 is in pink, with the RPB1
C-terminal tail in red; RPB2 is in cyan, RPB3 and RPB11 are in
green; subunits of RNAP II without counterparts in bacterial
RNAP (RPB5, RPB8, RPB9, RPB10, and RPB12) are in gray; the
active-center Mg2+ is in magenta. (e) Structural alignment of
 (cyan;
residues 9-81) and RPB6 (red; residues 80-138). (f) Sequences of
segments of the RNAP largest subunit that interact with (cyan;
residues 9-81) and RPB6 (red; residues 80-138). (f) Sequences of
segments of the RNAP largest subunit that interact with  (a) and
RBP6 (c). Conserved regions of the RNAP largest subunit are
indicated by lettered boxes (8). The amino acid substitutions in
E. coli rpoC^tsX and yeast RPB1-1 are indicated above the
aligned sequences. Sequences shown are, in order: T. aquaticus (a) and
RBP6 (c). Conserved regions of the RNAP largest subunit are
indicated by lettered boxes (8). The amino acid substitutions in
E. coli rpoC^tsX and yeast RPB1-1 are indicated above the
aligned sequences. Sequences shown are, in order: T. aquaticus
 '
(CAB65466), E. coli '
(CAB65466), E. coli  '
(RPOC_ECOLI), S. cerevisiae RPA1 (RPA1_YEAST), S. cerevisiae
RPB1 (RPB1_YEAST), and S. cerevisiae RPC1 (RPC1_YEAST). The dots
indicate amino acid identities. '
(RPOC_ECOLI), S. cerevisiae RPA1 (RPA1_YEAST), S. cerevisiae
RPB1 (RPB1_YEAST), and S. cerevisiae RPC1 (RPC1_YEAST). The dots
indicate amino acid identities.
|
 |
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.Albert,
I.Léger-Silvestre,
C.Normand,
M.K.Ostermaier,
J.Pérez-Fernández,
K.I.Panov,
J.C.Zomerdijk,
P.Schultz,
and
O.Gadal
(2011).
RNA polymerase I-specific subunits promote polymerase clustering to enhance the rRNA gene transcription cycle.
|
| |
J Cell Biol,
192,
277-293.
|
 |
|
|
|
|
 |
G.P.Doherty,
M.J.Fogg,
A.J.Wilkinson,
and
P.J.Lewis
(2010).
Small subunits of RNA polymerase: localization, levels and implications for core enzyme composition.
|
| |
Microbiology,
156,
3532-3543.
|
 |
|
|
|
|
 |
P.F.Gherardini,
G.Ausiello,
R.B.Russell,
and
M.Helmer-Citterich
(2010).
Modular architecture of nucleotide-binding pockets.
|
| |
Nucleic Acids Res,
38,
3809-3816.
|
 |
|
|
|
|
 |
W.J.Lane,
and
S.A.Darst
(2010).
Molecular evolution of multisubunit RNA polymerases: structural analysis.
|
| |
J Mol Biol,
395,
686-704.
|
 |
|
|
|
|
 |
A.Y.Mulkidjanian,
and
M.Y.Galperin
(2009).
On the origin of life in the Zinc world. 2. Validation of the hypothesis on the photosynthesizing zinc sulfide edifices as cradles of life on Earth.
|
| |
Biol Direct,
4,
27.
|
 |
|
|
|
|
 |
E.B.Johnston,
P.J.Lewis,
and
R.Griffith
(2009).
The interaction of Bacillus subtilis sigmaA with RNA polymerase.
|
| |
Protein Sci,
18,
2287-2297.
|
 |
|
|
|
|
 |
E.Nudler
(2009).
RNA polymerase active center: the molecular engine of transcription.
|
| |
Annu Rev Biochem,
78,
335-361.
|
 |
|
|
|
|
 |
J.D.Helmann
(2009).
RNA polymerase: a nexus of gene regulation.
|
| |
Methods,
47,
1-5.
|
 |
|
|
|
|
 |
P.Cramer,
K.J.Armache,
S.Baumli,
S.Benkert,
F.Brueckner,
C.Buchen,
G.E.Damsma,
S.Dengl,
S.R.Geiger,
A.J.Jasiak,
A.Jawhari,
S.Jennebach,
T.Kamenski,
H.Kettenberger,
C.D.Kuhn,
E.Lehmann,
K.Leike,
J.F.Sydow,
and
A.Vannini
(2008).
Structure of eukaryotic RNA polymerases.
|
| |
Annu Rev Biophys,
37,
337-352.
|
 |
|
|
|
|
 |
P.J.Lewis,
G.P.Doherty,
and
J.Clarke
(2008).
Transcription factor dynamics.
|
| |
Microbiology,
154,
1837-1844.
|
 |
|
|
|
|
 |
S.Borukhov,
and
E.Nudler
(2008).
RNA polymerase: the vehicle of transcription.
|
| |
Trends Microbiol,
16,
126-134.
|
 |
|
|
|
|
 |
S.P.Haugen,
W.Ross,
and
R.L.Gourse
(2008).
Advances in bacterial promoter recognition and its control by factors that do not bind DNA.
|
| |
Nat Rev Microbiol,
6,
507-519.
|
 |
|
|
|
|
 |
E.A.Lysenko
(2007).
Plant sigma factors and their role in plastid transcription.
|
| |
Plant Cell Rep,
26,
845-859.
|
 |
|
|
|
|
 |
S.Martínez-Calvillo,
A.Saxena,
A.Green,
A.Leland,
and
P.J.Myler
(2007).
Characterization of the RNA polymerase II and III complexes in Leishmania major.
|
| |
Int J Parasitol,
37,
491-502.
|
 |
|
|
|
|
 |
T.N.Nguyen,
B.Schimanski,
and
A.Günzl
(2007).
Active RNA polymerase I of Trypanosoma brucei harbors a novel subunit essential for transcription.
|
| |
Mol Cell Biol,
27,
6254-6263.
|
 |
|
|
|
|
 |
C.S.Pikaard
(2006).
Cell biology of the Arabidopsis nuclear siRNA pathway for RNA-directed chromatin modification.
|
| |
Cold Spring Harb Symp Quant Biol,
71,
473-480.
|
 |
|
|
|
|
 |
L.Yuste,
A.B.Hervás,
I.Canosa,
R.Tobes,
J.I.Jiménez,
J.Nogales,
M.M.Pérez-Pérez,
E.Santero,
E.Díaz,
J.L.Ramos,
V.de Lorenzo,
and
F.Rojo
(2006).
Growth phase-dependent expression of the Pseudomonas putida KT2440 transcriptional machinery analysed with a genome-wide DNA microarray.
|
| |
Environ Microbiol,
8,
165-177.
|
 |
|
|
|
|
 |
R.Mathew,
and
D.Chatterji
(2006).
The evolving story of the omega subunit of bacterial RNA polymerase.
|
| |
Trends Microbiol,
14,
450-455.
|
 |
|
|
|
|
 |
V.Trinh,
M.F.Langelier,
J.Archambault,
and
B.Coulombe
(2006).
Structural perspective on mutations affecting the function of multisubunit RNA polymerases.
|
| |
Microbiol Mol Biol Rev,
70,
12-36.
|
 |
|
|
|
|
 |
A.J.Smith,
and
N.J.Savery
(2005).
RNA polymerase mutants defective in the initiation of transcription-coupled DNA repair.
|
| |
Nucleic Acids Res,
33,
755-764.
|
 |
|
|
|
|
 |
A.Lewin,
M.Mayer,
J.Chusainow,
D.Jacob,
and
B.Appel
(2005).
Viral promoters can initiate expression of toxin genes introduced into Escherichia coli.
|
| |
BMC Biotechnol,
5,
19.
|
 |
|
|
|
|
 |
A.Travers,
and
G.Muskhelishvili
(2005).
DNA supercoiling - a global transcriptional regulator for enterobacterial growth?
|
| |
Nat Rev Microbiol,
3,
157-169.
|
 |
|
|
|
|
 |
C.E.Vrentas,
T.Gaal,
W.Ross,
R.H.Ebright,
and
R.L.Gourse
(2005).
Response of RNA polymerase to ppGpp: requirement for the omega subunit and relief of this requirement by DksA.
|
| |
Genes Dev,
19,
2378-2387.
|
 |
|
|
|
|
 |
E.P.Geiduschek,
and
M.Ouhammouch
(2005).
Archaeal transcription and its regulators.
|
| |
Mol Microbiol,
56,
1397-1407.
|
 |
|
|
|
|
 |
J.L.Knight,
V.Mekler,
J.Mukhopadhyay,
R.H.Ebright,
and
R.M.Levy
(2005).
Distance-restrained docking of rifampicin and rifamycin SV to RNA polymerase using systematic FRET measurements: developing benchmarks of model quality and reliability.
|
| |
Biophys J,
88,
925-938.
|
 |
|
|
|
|
 |
M.S.Bartlett
(2005).
Determinants of transcription initiation by archaeal RNA polymerase.
|
| |
Curr Opin Microbiol,
8,
677-684.
|
 |
|
|
|
|
 |
R.Mathew,
M.Ramakanth,
and
D.Chatterji
(2005).
Deletion of the gene rpoZ, encoding the omega subunit of RNA polymerase, in Mycobacterium smegmatis results in fragmentation of the beta' subunit in the enzyme assembly.
|
| |
J Bacteriol,
187,
6565-6570.
|
 |
|
|
|
|
 |
S.Abhiman,
and
E.L.Sonnhammer
(2005).
Large-scale prediction of function shift in protein families with a focus on enzymatic function.
|
| |
Proteins,
60,
758-768.
|
 |
|
|
|
|
 |
W.Ross,
and
R.L.Gourse
(2005).
Sequence-independent upstream DNA-alphaCTD interactions strongly stimulate Escherichia coli RNA polymerase-lacUV5 promoter association.
|
| |
Proc Natl Acad Sci U S A,
102,
291-296.
|
 |
|
|
|
|
 |
B.S.Chen,
and
M.Hampsey
(2004).
Functional interaction between TFIIB and the Rpb2 subunit of RNA polymerase II: implications for the mechanism of transcription initiation.
|
| |
Mol Cell Biol,
24,
3983-3991.
|
 |
|
|
|
|
 |
K.Nakagawa,
K.Hisatake,
Y.Imazawa,
A.Ishiguro,
M.Matsumoto,
L.Pape,
A.Ishihama,
and
Y.Nogi
(2003).
The fission yeast RPA51 is a functional homolog of the budding yeast A49 subunit of RNA polymerase I and required for maximizing transcription of ribosomal DNA.
|
| |
Genes Genet Syst,
78,
199-209.
|
 |
|
|
|
|
 |
K.S.Murakami,
and
S.A.Darst
(2003).
Bacterial RNA polymerases: the wholo story.
|
| |
Curr Opin Struct Biol,
13,
31-39.
|
 |
|
|
|
|
 |
M.F.Simeonov,
R.J.Bieber Urbauer,
J.M.Gilmore,
K.Adelman,
E.N.Brody,
A.Niedziela-Majka,
L.Minakhin,
T.Heyduk,
and
J.L.Urbauer
(2003).
Characterization of the interactions between the bacteriophage T4 AsiA protein and RNA polymerase.
|
| |
Biochemistry,
42,
7717-7726.
|
 |
|
|
|
|
 |
N.Opalka,
M.Chlenov,
P.Chacon,
W.J.Rice,
W.Wriggers,
and
S.A.Darst
(2003).
Structure and function of the transcription elongation factor GreB bound to bacterial RNA polymerase.
|
| |
Cell,
114,
335-345.
|
 |
|
|
|
|
 |
Q.Tan,
M.H.Prysak,
and
N.A.Woychik
(2003).
Loss of the Rpb4/Rpb7 subcomplex in a mutant form of the Rpb6 subunit shared by RNA polymerases I, II, and III.
|
| |
Mol Cell Biol,
23,
3329-3338.
|
 |
|
|
|
|
 |
T.Naryshkina,
A.Bruning,
O.Gadal,
and
K.Severinov
(2003).
Role of second-largest RNA polymerase I subunit Zn-binding domain in enzyme assembly.
|
| |
Eukaryot Cell,
2,
1046-1052.
|
 |
|
|
|
|
 |
G.Peyroche,
E.Levillain,
M.Siaut,
I.Callebaut,
P.Schultz,
A.Sentenac,
M.Riva,
and
C.Carles
(2002).
The A14-A43 heterodimer subunit in yeast RNA pol I and their relationship to Rpb4-Rpb7 pol II subunits.
|
| |
Proc Natl Acad Sci U S A,
99,
14670-14675.
|
 |
|
|
|
|
 |
H.Sakurai,
and
A.Ishihama
(2002).
Level of the RNA polymerase II in the fission yeast stays constant but phosphorylation of its carboxyl terminal domain varies depending on the phase and rate of cell growth.
|
| |
Genes Cells,
7,
273-284.
|
 |
|
|
|
|
 |
I.Kojima,
K.Kasuga,
M.Kobayashi,
A.Fukasawa,
S.Mizuno,
A.Arisawa,
and
H.Akagawa
(2002).
The rpoZ gene, encoding the RNA polymerase omega subunit, is required for antibiotic production and morphological differentiation in Streptomyces kasugaensis.
|
| |
J Bacteriol,
184,
6417-6423.
|
 |
|
|
|
|
 |
J.L.Craighead,
W.H.Chang,
and
F.J.Asturias
(2002).
Structure of yeast RNA polymerase II in solution: implications for enzyme regulation and interaction with promoter DNA.
|
| |
Structure,
10,
1117-1125.
|
 |
|
|
|
|
 |
M.N.Vassylyeva,
J.Lee,
S.I.Sekine,
O.Laptenko,
S.Kuramitsu,
T.Shibata,
Y.Inoue,
S.Borukhov,
D.G.Vassylyev,
and
S.Yokoyama
(2002).
Purification, crystallization and initial crystallographic analysis of RNA polymerase holoenzyme from Thermus thermophilus.
|
| |
Acta Crystallogr D Biol Crystallogr,
58,
1497-1500.
|
 |
|
|
|
|
 |
N.Bischler,
L.Brino,
C.Carles,
M.Riva,
H.Tschochner,
V.Mallouh,
and
P.Schultz
(2002).
Localization of the yeast RNA polymerase I-specific subunits.
|
| |
EMBO J,
21,
4136-4144.
|
 |
|
|
|
|
 |
P.Cramer
(2002).
Multisubunit RNA polymerases.
|
| |
Curr Opin Struct Biol,
12,
89-97.
|
 |
|
|
|
|
 |
S.A.Darst,
N.Opalka,
P.Chacon,
A.Polyakov,
C.Richter,
G.Zhang,
and
W.Wriggers
(2002).
Conformational flexibility of bacterial RNA polymerase.
|
| |
Proc Natl Acad Sci U S A,
99,
4296-4301.
|
 |
|
|
|
|
 |
V.Van Mullem,
E.Landrieux,
J.Vandenhaute,
and
P.Thuriaux
(2002).
Rpa12p, a conserved RNA polymerase I subunit with two functional domains.
|
| |
Mol Microbiol,
43,
1105-1113.
|
 |
|
|
|
|
 |
B.A.Young,
L.C.Anthony,
T.M.Gruber,
T.M.Arthur,
E.Heyduk,
C.Z.Lu,
M.M.Sharp,
T.Heyduk,
R.R.Burgess,
and
C.A.Gross
(2001).
A coiled-coil from the RNA polymerase beta' subunit allosterically induces selective nontemplate strand binding by sigma(70).
|
| |
Cell,
105,
935-944.
|
 |
|
|
|
|
 |
E.A.Campbell,
N.Korzheva,
A.Mustaev,
K.Murakami,
S.Nair,
A.Goldfarb,
and
S.A.Darst
(2001).
Structural mechanism for rifampicin inhibition of bacterial rna polymerase.
|
| |
Cell,
104,
901-912.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.F.Briand,
F.Navarro,
P.Rematier,
C.Boschiero,
S.Labarre,
M.Werner,
G.V.Shpakovski,
and
P.Thuriaux
(2001).
Partners of Rpb8p, a small subunit shared by yeast RNA polymerases I, II and III.
|
| |
Mol Cell Biol,
21,
6056-6065.
|
 |
|
|
|
|
 |
M.S.Paget,
V.Molle,
G.Cohen,
Y.Aharonowitz,
and
M.J.Buttner
(2001).
Defining the disulphide stress response in Streptomyces coelicolor A3(2): identification of the sigmaR regulon.
|
| |
Mol Microbiol,
42,
1007-1020.
|
 |
|
|
|
|
 |
P.Ghosh,
A.Ishihama,
and
D.Chatterji
(2001).
Escherichia coli RNA polymerase subunit omega and its N-terminal domain bind full-length beta' to facilitate incorporation into the alpha2beta subassembly.
|
| |
Eur J Biochem,
268,
4621-4627.
|
 |
|
|
|
|
 |
S.A.Darst
(2001).
Bacterial RNA polymerase.
|
| |
Curr Opin Struct Biol,
11,
155-162.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|

| |

