 |
PDBsum entry 1fe2

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1fe2
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.99.1
- prostaglandin-endoperoxide synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate + AH2 + 2 O2 = prostaglandin H2 + A + H2O
|
 |
 |
 |
 |
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate
Bound ligand (Het Group name = )
matches with 51.16% similarity
|
+
|
AH2
|
+
|
 2
×
O2
2
×
O2
|
=
|
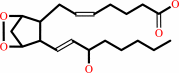 prostaglandin H2
prostaglandin H2
|
+
|
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
276:10358-10365
(2001)
|
|
PubMed id:
|
|
|
|

|
| |
|
Mutational and X-ray crystallographic analysis of the interaction of dihomo-gamma -linolenic acid with prostaglandin endoperoxide H synthases.
|
|
E.D.Thuresson,
M.G.Malkowski,
K.M.Lakkides,
C.J.Rieke,
A.M.Mulichak,
S.L.Ginell,
R.M.Garavito,
W.L.Smith.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Prostaglandin endoperoxide H synthases-1 and -2 (PGHSs) catalyze the committed
step in prostaglandin biosynthesis. Both isozymes can oxygenate a variety of
related polyunsaturated fatty acids. We report here the x-ray crystal structure
of dihomo-gamma-linolenic acid (DHLA) in the cyclooxygenase site of PGHS-1 and
the effects of active site substitutions on the oxygenation of DHLA, and we
compare these results to those obtained previously with arachidonic acid (AA).
DHLA is bound within the cyclooxygenase site in the same overall L-shaped
conformation as AA. C-1 and C-11 through C-20 are in the same positions for both
substrates, but the positions of C-2 through C-10 differ by up to 1.74 A. In
general, substitutions of active site residues caused parallel changes in the
oxygenation of both AA and DHLA. Two significant exceptions were Val-349 and
Ser-530. A V349A substitution caused an 800-fold decrease in the V(max)/K(m) for
DHLA but less than a 2-fold change with AA; kinetic evidence indicates that C-13
of DHLA is improperly positioned with respect to Tyr-385 in the V349A mutant
thereby preventing efficient hydrogen abstraction. Val-349 contacts C-5 of DHLA
and appears to serve as a structural bumper positioning the carboxyl half of
DHLA, which, in turn, positions properly the omega-half of this substrate. A
V349A substitution in PGHS-2 has similar, minor effects on the rates of
oxygenation of AA and DHLA. Thus, Val-349 is a major determinant of substrate
specificity for PGHS-1 but not for PGHS-2. Ser-530 also influences the substrate
specificity of PGHS-1; an S530T substitution causes 40- and 750-fold decreases
in oxygenation efficiencies for AA and DHLA, respectively.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig. 2. Comparison of the binding of AA and DHLA within
the cyclooxygenase active site. A stereo view of DHLA (red) and
AA (light blue) (7) bound within in the cyclooxygenase active
site channel of oPGHS-1. Active site residues are colored as in
Fig. 1. The absence of the C5/C6 double bond in DHLA allows for
greater conformational flexibility in the carboxyl half of the
substrates as compared with AA. This is reflected in the
1.1-Å r.m.s. deviation between carbon positions in DHLA
versus AA for C-1 to C-10. Additionally, the position of the C
 -2 atom of
Ile-523 (orange in DHLA versus blue in AA) and the O -2 atom of
Ile-523 (orange in DHLA versus blue in AA) and the O  atom on
Ser-530 (light green in DHLA versus magenta in AA) move to
accommodate DHLA in the active site. atom on
Ser-530 (light green in DHLA versus magenta in AA) move to
accommodate DHLA in the active site.
|
 |
Figure 3.
Fig. 3. Interactions between DHLA and cyclooxygenase
active site residues. A schematic diagram of the interactions
between DHLA and residues within the cyclooxygenase channel.
Every other carbon atom of DHLA is labeled, and the hydrogens
for C-13 have been modeled. All dashed lines represent
interactions within 4.0 Å between the specific side chain
atom of the protein and DHLA. Only 3 of the 62 contacts between
DHLA and cyclooxygenase channel residues are hydrophilic.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2001,
276,
10358-10365)
copyright 2001.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Y.Xiao,
Y.Gu,
P.Purwaha,
K.Ni,
B.Law,
S.Mallik,
and
S.Y.Qian
(2011).
Characterization of free radicals formed from COX-catalyzed DGLA peroxidation.
|
| |
Free Radic Biol Med,
50,
1163-1170.
|
 |
|
|
|
|
 |
A.L.Tsai,
and
R.J.Kulmacz
(2010).
Prostaglandin H synthase: resolved and unresolved mechanistic issues.
|
| |
Arch Biochem Biophys,
493,
103-124.
|
 |
|
|
|
|
 |
M.Koszelak-Rosenblum,
A.C.Krol,
D.M.Simmons,
C.C.Goulah,
L.Wroblewski,
and
M.G.Malkowski
(2008).
His-311 and Arg-559 are key residues involved in fatty acid oxygenation in pathogen-inducible oxygenase.
|
| |
J Biol Chem,
283,
24962-24971.
|
 |
|
|
|
|
 |
C.E.Rogge,
B.Ho,
W.Liu,
R.J.Kulmacz,
and
A.L.Tsai
(2006).
Role of Tyr348 in Tyr385 radical dynamics and cyclooxygenase inhibitor interactions in prostaglandin H synthase-2.
|
| |
Biochemistry,
45,
523-532.
|
 |
|
|
|
|
 |
C.Yuan,
C.J.Rieke,
G.Rimon,
B.A.Wingerd,
and
W.L.Smith
(2006).
Partnering between monomers of cyclooxygenase-2 homodimers.
|
| |
Proc Natl Acad Sci U S A,
103,
6142-6147.
|
 |
|
|
|
|
 |
K.E.Furse,
D.A.Pratt,
N.A.Porter,
and
T.P.Lybrand
(2006).
Molecular dynamics simulations of arachidonic acid complexes with COX-1 and COX-2: insights into equilibrium behavior.
|
| |
Biochemistry,
45,
3189-3205.
|
 |
|
|
|
|
 |
H.Park,
and
S.Lee
(2005).
Free energy perturbation approach to the critical assessment of selective cyclooxygenase-2 inhibitors.
|
| |
J Comput Aided Mol Des,
19,
17-31.
|
 |
|
|
|
|
 |
R.G.Huff,
E.Bayram,
H.Tan,
S.T.Knutson,
M.H.Knaggs,
A.B.Richon,
P.Santago,
and
J.S.Fetrow
(2005).
Chemical and structural diversity in cyclooxygenase protein active sites.
|
| |
Chem Biodivers,
2,
1533-1552.
|
 |
|
|
|
|
 |
R.J.Kulmacz,
W.A.van der Donk,
and
A.L.Tsai
(2003).
Comparison of the properties of prostaglandin H synthase-1 and -2.
|
| |
Prog Lipid Res,
42,
377-404.
|
 |
|
|
|
|
 |
R.M.Garavito,
and
A.M.Mulichak
(2003).
The structure of mammalian cyclooxygenases.
|
| |
Annu Rev Biophys Biomol Struct,
32,
183-206.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

