 |
PDBsum entry 5foc
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Crystal structure of the p.Falciparum cytosolic leucyl-tRNA synthetase editing domain (space group p21)
|
|
Structure:
|
 |
Leucyl-tRNA synthetase. Chain: a, b. Fragment: editing domain, unp residues 272-687. Engineered: yes. Mutation: yes. Other_details: deletion of residues 475-519, c273s substitution to prevent disulfide bonds, gam inserted at n- term expression vector
|
|
Source:
|
 |
Plasmodium falciparum. Organism_taxid: 36329. Strain: 3d7. Expressed in: escherichia coli k-12. Expression_system_taxid: 83333. Expression_system_variant: de3 ril.
|
|
Resolution:
|
 |
|
1.50Å
|
R-factor:
|
0.185
|
R-free:
|
0.234
|
|
|
Authors:
|
 |
A.Palencia,E.Sonoiki,D.Guo,V.Ahyong,C.Dong,X.Li,V.S.Hernandez,J.Gut, J.Legac,R.Cooper,M.R.K.Alley,Y.R.Freund,J.Derisi,S.Cusack, P.J.Rosenthal
|
|
Key ref:
|
 |
E.Sonoiki
et al.
(2016).
Antimalarial Benzoxaboroles Target Plasmodium falciparum Leucyl-tRNA Synthetase.
Antimicrob Agents Chemother,
60,
4886-4895.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
19-Nov-15
|
Release date:
|
22-Jun-16
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
C6KT64
(C6KT64_PLAF7) -
leucine--tRNA ligase from Plasmodium falciparum (isolate 3D7)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
1447 a.a.
297 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.1.1.4
- leucine--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Leu) + L-leucine + ATP = L-leucyl-tRNA(Leu) + AMP + diphosphate
|
 |
 |
 |
 |
 |
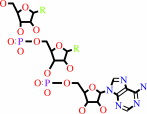 tRNA(Leu)
tRNA(Leu)
|
+
|
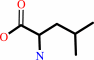 L-leucine
L-leucine
|
+
|
 ATP
ATP
|
=
|
 L-leucyl-tRNA(Leu)
L-leucyl-tRNA(Leu)
|
+
|
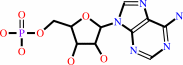 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Antimicrob Agents Chemother
60:4886-4895
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Antimalarial Benzoxaboroles Target Plasmodium falciparum Leucyl-tRNA Synthetase.
|
|
E.Sonoiki,
A.Palencia,
D.Guo,
V.Ahyong,
C.Dong,
X.Li,
V.S.Hernandez,
Y.K.Zhang,
W.Choi,
J.Gut,
J.Legac,
R.Cooper,
M.R.Alley,
Y.R.Freund,
J.DeRisi,
S.Cusack,
P.J.Rosenthal.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
There is a need for new antimalarials, ideally with novel mechanisms of action.
Benzoxaboroles have been shown to be active against bacteria, fungi, and
trypanosomes. Therefore, we investigated the antimalarial activity and mechanism
of action of 3-aminomethyl benzoxaboroles against Plasmodium falciparum Two
3-aminomethyl compounds, AN6426 and AN8432, demonstrated good potency against
cultured multidrug-resistant (W2 strain) P. falciparum (50% inhibitory
concentration [IC50] of 310 nM and 490 nM, respectively) and efficacy against
murine Plasmodium berghei infection when administered orally once daily for 4
days (90% effective dose [ED90], 7.4 and 16.2 mg/kg of body weight,
respectively). To characterize mechanisms of action, we selected parasites with
decreased drug sensitivity by culturing with stepwise increases in concentration
of AN6426. Resistant clones were characterized by whole-genome sequencing. Three
generations of resistant parasites had polymorphisms in the predicted editing
domain of the gene encoding a P. falciparum leucyl-tRNA synthetase (LeuRS;
PF3D7_0622800) and in another gene (PF3D7_1218100), which encodes a protein of
unknown function. Solution of the structure of the P. falciparum LeuRS editing
domain suggested key roles for mutated residues in LeuRS editing. Short
incubations with AN6426 and AN8432, unlike artemisinin, caused dose-dependent
inhibition of [(14)C]leucine incorporation by cultured wild-type, but not
resistant, parasites. The growth of resistant, but not wild-type, parasites was
impaired in the presence of the unnatural amino acid norvaline, consistent with
a loss of LeuRS editing activity in resistant parasites. In summary, the
benzoxaboroles AN6426 and AN8432 offer effective antimalarial activity and act,
at least in part, against a novel target, the editing domain of P. falciparum
LeuRS.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

