 |
PDBsum entry 4zya
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
The n-terminal extension domain of human asparaginyl-tRNA synthetase
|
|
Structure:
|
 |
Asparagine--tRNA ligase, cytoplasmic. Chain: a, b. Fragment: unp residues 4-77. Synonym: asparaginyl-tRNA synthetase,asnrs. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: nars. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
1.65Å
|
R-factor:
|
0.211
|
R-free:
|
0.254
|
|
|
Authors:
|
 |
J.S.Park,M.C.Park,P.Goughnour,H.S.Kim,S.J.Kim,H.J.Kim,S.H.Kim,B.W.Han
|
|
Key ref:
|
 |
J.S.Park
et al.
(2018).
Unique N-terminal extension domain of human asparaginyl-tRNA synthetase elicits CCR3-mediated chemokine activity.
Int J Biol Macromol,
120,
835-845.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
21-May-15
|
Release date:
|
25-May-16
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.6.1.1.22
- asparagine--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Asn) + L-asparagine + ATP = L-asparaginyl-tRNA(Asn) + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
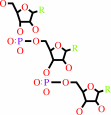 tRNA(Asn)
tRNA(Asn)
|
+
|
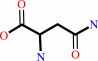 L-asparagine
L-asparagine
|
+
|
 ATP
ATP
|
=
|
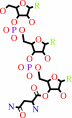 L-asparaginyl-tRNA(Asn)
L-asparaginyl-tRNA(Asn)
|
+
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Int J Biol Macromol
120:835-845
(2018)
|
|
PubMed id:
|
|
|
|

|
| |
|
Unique N-terminal extension domain of human asparaginyl-tRNA synthetase elicits CCR3-mediated chemokine activity.
|
|
J.S.Park,
M.C.Park,
K.Y.Lee,
P.C.Goughnour,
S.J.Jeong,
H.S.Kim,
H.J.Kim,
B.J.Lee,
S.Kim,
B.W.Han.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Asparaginyl-tRNA synthetase (NRS) is not only essential in protein translation
but also associated with autoimmune diseases. Particularly, patients with
antibodies that recognize NRS often develop interstitial lung disease (ILD).
However, the underlying mechanism of how NRS is recognized by immune cells and
provokes inflammatory responses is not well-understood. Here, we found that the
crystal structure of the unique N-terminal extension domain of human NRS (named
as UNE-N, where -N denotes NRS) resembles that of the chemotactic N-terminal
domain of NRS from a filarial nematode, Brugia malayi, which recruits and
activates specific immune cells by interacting with CXC chemokine receptor 1 and
2. UNE-N induced migration of CC chemokine receptor 3 (CCR3)-expressing cells.
The chemokine activity of UNE-N was significantly reduced by suppressing CCR3
expression with CCR3-targeting siRNA, and the loop3 region of UNE-N was shown to
interact mainly with the extracellular domains of CCR3 in nuclear magnetic
resonance perturbation experiments. Based on these results, evolutionarily
acquired UNE-N elicits chemokine activities that would promote NRS-CCR3-mediated
proinflammatory signaling in ILD.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

