 |
PDBsum entry 4q5e

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Unknown function, protein binding
|
PDB id
|
|

|

|
4q5e
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|
 168 a.a.
168 a.a.
|
 |

|

|

|

|

|

|

|
 76 a.a.
76 a.a.
|
 |

|

|

|

|

|

|

|
 155 a.a.
155 a.a.
|
 |

|

|
|
|
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Unknown function, protein binding
|
 |
|
Title:
|
 |
Shigella effector kinase ospg bound to e2-ub ubch7-ub conjugate
|
|
Structure:
|
 |
Protein kinase ospg. Chain: a. Fragment: unp residues 26-193. Synonym: effector protein ospg. Engineered: yes. Polyubiquitin. Chain: b. Synonym: ubiquitin. Engineered: yes.
|
|
Source:
|
 |
Shigella sonnei ss046. Organism_taxid: 300269. Strain: 2457t. Gene: ospg, sson_p170. Expressed in: escherichia coli. Expression_system_taxid: 469008. Saccharomyces cerevisiae. Baker's yeast. Organism_taxid: 559292.
|
|
Resolution:
|
 |
|
1.87Å
|
R-factor:
|
0.187
|
R-free:
|
0.232
|
|
|
Authors:
|
 |
M.Cygler,A.M.Grishin,Montreal-Kingston Bacterial Structural Genomics Initiative (Bsgi)
|
|
Key ref:
|
 |
A.M.Grishin
et al.
(2014).
Structural basis for the inhibition of host protein ubiquitination by Shigella effector kinase OspG.
Structure,
22,
878-888.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
16-Apr-14
|
Release date:
|
02-Jul-14
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q3YTH2
(OSPG_SHISS) -
Protein kinase OspG from Shigella sonnei (strain Ss046)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
196 a.a.
168 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chain A:
E.C.2.7.-.-
|
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chain B:
E.C.6.3.2.19
- Transferred entry: 2.3.2.23, 2.3.2.27 and 6.2.1.45.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + ubiquitin + protein lysine = AMP + diphosphate + protein N-ubiquityllysine
|
 |
 |
 |
 |
 |
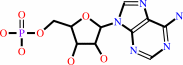
 ATP
ATP
|
+
|
ubiquitin
|
+
|
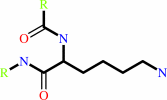 protein lysine
protein lysine
|
=
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
protein N-ubiquityllysine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
Chain C:
E.C.2.3.2.23
- E2 ubiquitin-conjugating enzyme.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
S-ubiquitinyl-[E1 ubiquitin-activating enzyme]-L-cysteine + [E2 ubiquitin-conjugating enzyme]-L-cysteine = [E1 ubiquitin-activating enzyme]-L-cysteine + S-ubiquitinyl-[E2 ubiquitin-conjugating enzyme]-L- cysteine
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
22:878-888
(2014)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis for the inhibition of host protein ubiquitination by Shigella effector kinase OspG.
|
|
A.M.Grishin,
T.E.Condos,
K.R.Barber,
F.X.Campbell-Valois,
C.Parsot,
G.S.Shaw,
M.Cygler.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Shigella invasion of its human host is assisted by T3SS-delivered effector
proteins. The OspG effector kinase binds ubiquitin and ubiquitin-loaded
E2-conjugating enzymes, including UbcH5b and UbcH7, and attenuates the host
innate immune NF-kB signaling. We present the structure of OspG bound to the
UbcH7∼Ub conjugate. OspG has a minimal kinase fold lacking the activation loop
of regulatory kinases. UbcH7∼Ub binds OspG at sites remote from the kinase
active site, yet increases its kinase activity. The ubiquitin is positioned in
the "open" conformation with respect to UbcH7 using its I44 patch to
interact with the C terminus of OspG. UbcH7 binds to OspG using two conserved
loops essential for E3 ligase recruitment. The interaction of the UbcH7∼Ub
with OspG is remarkably similar to the interaction of an E2∼Ub with a HECT E3
ligase. OspG interferes with the interaction of UbcH7 with the E3 parkin and
inhibits the activity of the E3.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| |

