 |
PDBsum entry 3wyl

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/hydrolase inhibitor
|
PDB id
|
|

|

|
3wyl
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase/hydrolase inhibitor
|
 |
|
Title:
|
 |
Crystal structure of the catalytic domain of pde10a complexed with 5- methoxy-3-(1-phenyl-1h-pyrazol-5-yl)-1-(3-(trifluoromethyl)phenyl) pyridazin-4(1h)-one
|
|
Structure:
|
 |
Camp and camp-inhibited cgmp 3',5'-cyclic phosphodiesterase 10a. Chain: a, b. Fragment: catalytic domain, unp residues 442-779. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: pde10a. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
2.68Å
|
R-factor:
|
0.203
|
R-free:
|
0.261
|
|
|
Authors:
|
 |
H.Oki,Y.Hayano
|
|
Key ref:
|
 |
J.Kunitomo
et al.
(2014).
Discovery of 1-[2-fluoro-4-(1H-pyrazol-1-yl)phenyl]-5-methoxy-3-(1-phenyl-1H-pyrazol-5-yl)pyridazin-4(1H)-one (TAK-063), a highly potent, selective, and orally active phosphodiesterase 10A (PDE10A) inhibitor.
J Med Chem,
57,
9627-9643.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
01-Sep-14
|
Release date:
|
19-Nov-14
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9Y233
(PDE10_HUMAN) -
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
1055 a.a.
315 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.4.17
- 3',5'-cyclic-nucleotide phosphodiesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a nucleoside 3',5'-cyclic phosphate + H2O = a nucleoside 5'-phosphate + H+
|
 |
 |
 |
 |
 |
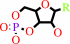 nucleoside 3',5'-cyclic phosphate
nucleoside 3',5'-cyclic phosphate
|
+
|
H2O
|
=
|
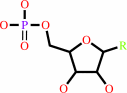 nucleoside 5'-phosphate
nucleoside 5'-phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
57:9627-9643
(2014)
|
|
PubMed id:
|
|
|
|

|
| |
|
Discovery of 1-[2-fluoro-4-(1H-pyrazol-1-yl)phenyl]-5-methoxy-3-(1-phenyl-1H-pyrazol-5-yl)pyridazin-4(1H)-one (TAK-063), a highly potent, selective, and orally active phosphodiesterase 10A (PDE10A) inhibitor.
|
|
J.Kunitomo,
M.Yoshikawa,
M.Fushimi,
A.Kawada,
J.F.Quinn,
H.Oki,
H.Kokubo,
M.Kondo,
K.Nakashima,
N.Kamiguchi,
K.Suzuki,
H.Kimura,
T.Taniguchi.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
A novel series of pyridazinone-based phosphodiesterase 10A (PDE10A) inhibitors
were synthesized. Our optimization efforts using structure-based drug design
(SBDD) techniques on the basis of the X-ray crystal structure of PDE10A in
complex with hit compound 1 (IC50 = 23 nM; 110-fold selectivity over other PDEs)
led to the identification of
1-[2-fluoro-4-(1H-pyrazol-1-yl)phenyl]-5-methoxy-3-(1-phenyl-1H-pyrazol-5-yl)pyridazin-4(1H)-one
(27h). Compound 27h has potent inhibitory activity (IC50 = 0.30 nM), excellent
selectivity (>15000-fold selectivity over other PDEs), and favorable
pharmacokinetics, including high brain penetration, in mice. Oral administration
of compound 27h to mice elevated striatal 3',5'-cyclic adenosine monophosphate
(cAMP) and 3',5'-cyclic guanosine monophosphate (cGMP) levels at 0.3 mg/kg and
showed potent suppression of phencyclidine (PCP)-induced hyperlocomotion at a
minimum effective dose (MED) of 0.3 mg/kg. Compound 27h (TAK-063) is currently
being evaluated in clinical trials for the treatment of schizophrenia.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

