 |
PDBsum entry 1scw

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.4.16.4
- serine-type D-Ala-D-Ala carboxypeptidase.
|
|
 |
 |
 |
 |
 |
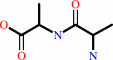
Reaction:
|
 |
D-alanyl-D-alanine + H2O = 2 D-alanine
|
 |
 |
 |
 |
 |
|
+
|
|
=
|
2
×
Bound ligand (Het Group name = )
matches with 50.00% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
43:7046-7053
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Toward better antibiotics: crystallographic studies of a novel class of DD-peptidase/beta-lactamase inhibitors.
|
|
N.R.Silvaggi,
K.Kaur,
S.A.Adediran,
R.F.Pratt,
J.A.Kelly.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Beta-lactam antibiotics are vital weapons in the treatment of bacterial
infections, but their future is under increasing threat from beta-lactamases.
These bacterial enzymes hydrolyze and inactivate beta-lactam antibiotics,
rendering the host cell resistant to the bactericidal effects of the drugs.
Nevertheless, the bacterial D-alanyl-D-alanine transpeptidases (DD-peptidases),
the killing targets of beta-lactams, remain attractive targets for antibiotic
compounds. Cyclic acyl phosph(on)ates have been developed and investigated as
potential inhibitors of both transpeptidases and beta-lactamases. The X-ray
crystal structures of the complexes of the Streptomyces strain R61 DD-peptidase
inhibited by a bicyclic
[1-hydroxy-4,5-benzo-2,6-dioxaphosphorinanone(3)-1-oxide] and a monocyclic
[1-hydroxy-4-phenyl-2,6-dioxaphosphorinanone(3)-1-oxide] acyl phosphate were
determined to investigate the mode of action of these novel inhibitors. The
structures show, first, that these inhibitors form covalent bonds with the
active site serine residue of the enzyme and that the refractory complexes thus
formed are phosphoryl-enzyme species rather than acyl enzymes. The complexes are
long-lived largely because, after ring opening, the ligands adopt conformations
that cannot directly recyclize, the latter a phenomenon previously observed with
cyclic acyl phosph(on)ates. While the two inhibitors bind in nearly identical
conformations, the phosphoryl-enzyme complex formed from the monocyclic compound
is significantly less mobile than that formed from the bicyclic compound.
Despite this difference, the complex with the bicyclic compound breaks down to
regenerate free enzyme somewhat more slowly than that of the monocyclic. This
may be because of steric problems associated with the reorientation of the
larger bicyclic ligand required for reactivation. The structures are strikingly
different in the orientation of the phosphoryl moiety from those generated using
more specific phosph(on)ates. Models of the noncovalent complexes of the
monocyclic compound with the R61 DD-peptidase and a structurally very similar
class C beta-lactamase suggest reasons why the former enzyme is phosphorylated
by this compound, while the latter is acylated. Finally, this paper provides
information that will help in the design of additional DD-peptidase inhibitors
with the potential to serve as leads in the development of novel antibiotics.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
N.Rhazi,
M.Delmarcelle,
E.Sauvage,
F.Jacquemotte,
K.Devriendt,
V.Tallon,
L.Ghosez,
and
J.M.Frère
(2005).
Specificity and reversibility of the transpeptidation reaction catalyzed by the Streptomyces R61 D-Ala-D-Ala peptidase.
|
| |
Protein Sci,
14,
2922-2928.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

