 |
PDBsum entry 1cps

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
HydrolasE(C-terminal peptidase)
|
PDB id
|
|

|

|
1cps
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.4.17.1
- carboxypeptidase A.
|
|
 |
 |
 |
 |
 |
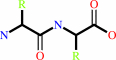
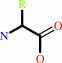
Reaction:
|
 |
Peptidyl-L-amino acid + H2O = peptide + L-amino acid
|
 |
 |
 |
 |
 |
|
+
|
|
=
|
|
+
|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Biol Chem
267:19192-19197
(1992)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural comparison of sulfodiimine and sulfonamide inhibitors in their complexes with zinc enzymes.
|
|
A.M.Cappalonga,
R.S.Alexander,
D.W.Christianson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The three-dimensional structure of (L(-)-2-carboxy-3-phenylpropyl)
methylsulfodiimine in its complex with the zinc metalloenzyme carboxypeptidase A
has been determined at 2.25-A resolution by x-ray crystallographic methods. This
is the first example of a sulfodiimine-containing inhibitor binding to a zinc
enzyme, and the structure of the enzyme-inhibitor complex reveals that the
tetrahedral sulfodiimine group coordinates to the active site zinc ion in
unidentate fashion. The zinc-coordinated nitrogen atom of the sulfodiimine group
is also within hydrogen bonding distance to active site base Glu-270;
presumably, the sulfodiimine is ionized and accepts a hydrogen bond from
protonated Glu-270. The other sulfodiimine nitrogen accepts a hydrogen bond from
Arg-127, and the inhibitor binds as a possible analogue of the tetrahedral
transition state (or intermediate) in a promoted water pathway for peptide
hydrolysis. The unidentate sulfodiimine-zinc binding mode observed in this
enzyme-inhibitor complex is reminiscent of that observed in sulfonamide
complexes with the zinc metalloenzyme carbonic anhydrase II, and the structural
features of sulfodiimine- and sulfonamide-zinc interactions exhibit important
similarities among recently determined structures of enzyme-inhibitor complexes:
ionized nitrogens bind to zinc in each structure, and these nitrogens are
engaged in hydrogen bond interactions with neighboring enzyme residues.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.M.van Aalten,
C.R.Chong,
and
L.Joshua-Tor
(2000).
Crystal structure of carboxypeptidase A complexed with D-cysteine at 1.75 A - inhibitor-induced conformational changes.
|
| |
Biochemistry,
39,
10082-10089.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.A.Laskowski,
N.M.Luscombe,
M.B.Swindells,
and
J.M.Thornton
(1996).
Protein clefts in molecular recognition and function.
|
| |
Protein Sci,
5,
2438-2452.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

