 |
PDBsum entry 6acb

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/hydrolase inhibitor
|
PDB id
|
|

|

|
6acb
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase/hydrolase inhibitor
|
 |
|
Title:
|
 |
Crystal structure of pde5 in complex with inhibitor lw1805
|
|
Structure:
|
 |
Cgmp-specific 3',5'-cyclic phosphodiesterase. Chain: a. Synonym: cgmp-binding cgmp-specific phosphodiesterase,cgb-pde. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: pde5a, pde5. Expressed in: escherichia coli bl21. Expression_system_taxid: 511693.
|
|
Resolution:
|
 |
|
2.80Å
|
R-factor:
|
0.201
|
R-free:
|
0.268
|
|
|
Authors:
|
 |
D.Wu,Y.D.Huang,Y.Y.Huang,H.B.Luo
|
|
Key ref:
|
 |
D.Wu
et al.
(2018).
Optimization of Chromeno[2,3- c]pyrrol-9(2 H)-ones as Highly Potent, Selective, and Orally Bioavailable PDE5 Inhibitors: Structure-Activity Relationship, X-ray Crystal Structure, and Pharmacodynamic Effect on Pulmonary Arterial Hypertension.
J Med Chem,
61,
8468-8473.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
26-Jul-18
|
Release date:
|
19-Sep-18
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
O76074
(PDE5A_HUMAN) -
cGMP-specific 3',5'-cyclic phosphodiesterase from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
875 a.a.
308 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.4.35
- 3',5'-cyclic-GMP phosphodiesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
3',5'-cyclic GMP + H2O = GMP + H+
|
 |
 |
 |
 |
 |
 3',5'-cyclic GMP
3',5'-cyclic GMP
|
+
|
H2O
|
=
|
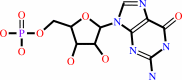 GMP
GMP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
61:8468-8473
(2018)
|
|
PubMed id:
|
|
|
|

|
| |
|
Optimization of Chromeno[2,3- c]pyrrol-9(2 H)-ones as Highly Potent, Selective, and Orally Bioavailable PDE5 Inhibitors: Structure-Activity Relationship, X-ray Crystal Structure, and Pharmacodynamic Effect on Pulmonary Arterial Hypertension.
|
|
D.Wu,
Y.Huang,
Y.Chen,
Y.Y.Huang,
H.Geng,
T.Zhang,
C.Zhang,
Z.Li,
L.Guo,
J.Chen,
H.B.Luo.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
To further explore the structure-activity relationship around the chromeno[2,3-
c]pyrrol-9(2 H)-one scaffold, 19 derivatives as inhibitors against PDE5 were
discovered. The most potent inhibitor 3 has an IC50 of 0.32 nM with
remarkable selectivity and druglike profile. Oral administration of 3 (1.25
mg/kg) caused comparable therapeutic effects to sildenafil (10.0 mg/kg) against
pulmonary arterial hypertension. Further, different binding patterns from
sildenafil were revealed in cocrystal structures, which provide structural
templates for discovery of highly potent PDE5 inhibitors.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

