 |
PDBsum entry 1sqc

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.4.2.1.129
- squalene--hopanol cyclase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
squalene + H2O = hopan-22-ol
|
 |
 |
 |
 |
 |
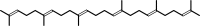 squalene
squalene
|
+
|
H2O
|
=
|
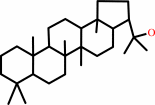 hopan-22-ol
hopan-22-ol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.5.4.99.17
- squalene--hopene cyclase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
squalene = hop-2229-ene
|
 |
 |
 |
 |
 |
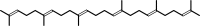 squalene
squalene
|
=
|
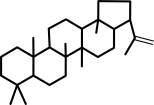 hop-22(29)-ene
hop-22(29)-ene
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Science
277:1811-1815
(1997)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure and function of a squalene cyclase.
|
|
K.U.Wendt,
K.Poralla,
G.E.Schulz.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of squalene-hopene cyclase from Alicyclobacillus
acidocaldarius was determined at 2.9 angstrom resolution. The mechanism and
sequence of this cyclase are closely related to those of 2,3-oxidosqualene
cyclases that catalyze the cyclization step in cholesterol biosynthesis. The
structure reveals a membrane protein with membrane-binding characteristics
similar to those of prostaglandin-H2 synthase, the only other reported protein
of this type. The active site of the enzyme is located in a large central cavity
that is of suitable size to bind squalene in its required conformation and that
is lined by aromatic residues. The structure supports a mechanism in which the
acid starting the reaction by protonating a carbon-carbon double bond is an
aspartate that is coupled to a histidine. Numerous surface alpha helices are
connected by characteristic QW-motifs (Q is glutamine and W is tryptophan) that
tighten the protein structure, possibly for absorbing the reaction energy
without structural damage.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. The proposed reaction steps in squalene-hopene
cyclases involving carbocationic intermediates. The general acid
B[1]:H protonates (H) squalene at C3, whereas the general base
B[2] deprotonates at C29 of the hopenyl cation. In a side
reaction, the cation is hydroxylated^ forming hopan-22-ol.
|
 |
Figure 5.
Fig. 5. The color-coded surface representations (30) with
nonpolar (yellow), positive (blue), and negative (red) areas.
(A) View similar to Fig. 2 but rotated around a vertical axis
and^ sliced. The cutting plane (checked) opens the large
internal cavity with the bound inhibitor LDAO. The nonpolar
channel runs to the^ left, opening into a nonpolar plateau. The
channel constriction (C) appears closed, but it is mobile enough
to be readily opened. At the upper left, hopane (two views) is
shown at scale. (B) View similar to Fig. 2 directly onto the
1600 Å2 nonpolar plateau with the channel entrance (E) at
its center and two nonpolar side chains pointing to the outside.
This is the only large nonpolar region on the surface.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the AAAs:
Science
(1997,
277,
1811-1815)
copyright 1997.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
G.E.Schulz
(2011).
A new classification of membrane protein crystals.
|
| |
J Mol Biol,
407,
640-646.
|
 |
|
|
|
|
 |
M.Köksal,
Y.Jin,
R.M.Coates,
R.Croteau,
and
D.W.Christianson
(2011).
Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis.
|
| |
Nature,
469,
116-120.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.D.Kiser,
and
K.Palczewski
(2010).
Membrane-binding and enzymatic properties of RPE65.
|
| |
Prog Retin Eye Res,
29,
428-442.
|
 |
|
|
|
|
 |
R.Cao,
Y.Zhang,
F.M.Mann,
C.Huang,
D.Mukkamala,
M.P.Hudock,
M.E.Mead,
S.Prisic,
K.Wang,
F.Y.Lin,
T.K.Chang,
R.J.Peters,
and
E.Oldfield
(2010).
Diterpene cyclases and the nature of the isoprene fold.
|
| |
Proteins,
78,
2417-2432.
|
 |
|
|
|
|
 |
R.R.Knowles,
and
E.N.Jacobsen
(2010).
Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts.
|
| |
Proc Natl Acad Sci U S A,
107,
20678-20685.
|
 |
|
|
|
|
 |
R.R.Knowles,
S.Lin,
and
E.N.Jacobsen
(2010).
Enantioselective thiourea-catalyzed cationic polycyclizations.
|
| |
J Am Chem Soc,
132,
5030-5032.
|
 |
|
|
|
|
 |
G.P.Ghimire,
T.J.Oh,
H.C.Lee,
and
J.K.Sohng
(2009).
Squalene-hopene cyclase (Spterp25) from Streptomyces peucetius: sequence analysis, expression and functional characterization.
|
| |
Biotechnol Lett,
31,
565-569.
|
 |
|
|
|
|
 |
K.Y.Wong,
J.P.Richard,
and
J.Gao
(2009).
Theoretical analysis of kinetic isotope effects on proton transfer reactions between substituted alpha-methoxystyrenes and substituted acetic acids.
|
| |
J Am Chem Soc,
131,
13963-13971.
|
 |
|
|
|
|
 |
L.K.Ranzer,
T.B.Brück,
W.M.Brück,
J.V.Lopez,
and
R.G.Kerr
(2009).
A new prokaryotic farnesyldiphosphate synthase from the octocoral eunicea fusca: differential display, inverse PCR, cloning, and characterization.
|
| |
Mar Biotechnol (NY),
11,
62-73.
|
 |
|
|
|
|
 |
T.Frickey,
and
E.Kannenberg
(2009).
Phylogenetic analysis of the triterpene cyclase protein family in prokaryotes and eukaryotes suggests bidirectional lateral gene transfer.
|
| |
Environ Microbiol,
11,
1224-1241.
|
 |
|
|
|
|
 |
Y.Wang,
R.I.Sadreyev,
and
N.V.Grishin
(2009).
PROCAIN: protein profile comparison with assisting information.
|
| |
Nucleic Acids Res,
37,
3522-3530.
|
 |
|
|
|
|
 |
D.W.Christianson
(2008).
Unearthing the roots of the terpenome.
|
| |
Curr Opin Chem Biol,
12,
141-150.
|
 |
|
|
|
|
 |
J.D.King,
E.F.Mulrooney,
E.Vinogradov,
B.Kneidinger,
K.Mead,
and
J.S.Lam
(2008).
lfnA from Pseudomonas aeruginosa O12 and wbuX from Escherichia coli O145 encode membrane-associated proteins and are required for expression of 2,6-dideoxy-2-acetamidino-L-galactose in lipopolysaccharide O antigen.
|
| |
J Bacteriol,
190,
1671-1679.
|
 |
|
|
|
|
 |
J.L.Kirschvink,
and
R.E.Kopp
(2008).
Palaeoproterozoic ice houses and the evolution of oxygen-mediating enzymes: the case for a late origin of photosystem II.
|
| |
Philos Trans R Soc Lond B Biol Sci,
363,
2755-2765.
|
 |
|
|
|
|
 |
R.P.McAndrew,
Y.Wang,
A.W.Mohsen,
M.He,
J.Vockley,
and
J.J.Kim
(2008).
Structural basis for substrate fatty acyl chain specificity: crystal structure of human very-long-chain acyl-CoA dehydrogenase.
|
| |
J Biol Chem,
283,
9435-9443.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Bosak,
R.M.Losick,
and
A.Pearson
(2008).
A polycyclic terpenoid that alleviates oxidative stress.
|
| |
Proc Natl Acad Sci U S A,
105,
6725-6729.
|
 |
|
|
|
|
 |
A.Pearson,
S.R.Flood Page,
T.L.Jorgenson,
W.W.Fischer,
and
M.B.Higgins
(2007).
Novel hopanoid cyclases from the environment.
|
| |
Environ Microbiol,
9,
2175-2188.
|
 |
|
|
|
|
 |
F.S.Mathews,
M.M.Gordon,
Z.Chen,
K.R.Rajashankar,
S.E.Ealick,
D.H.Alpers,
and
N.Sukumar
(2007).
Crystal structure of human intrinsic factor: cobalamin complex at 2.6-A resolution.
|
| |
Proc Natl Acad Sci U S A,
104,
17311-17316.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
I.Abe
(2007).
Enzymatic synthesis of cyclic triterpenes.
|
| |
Nat Prod Rep,
24,
1311-1331.
|
 |
|
|
|
|
 |
R.Rasteiro,
and
J.B.Pereira-Leal
(2007).
Multiple domain insertions and losses in the evolution of the Rab prenylation complex.
|
| |
BMC Evol Biol,
7,
140.
|
 |
|
|
|
|
 |
S.Prisic,
J.Xu,
R.M.Coates,
and
R.J.Peters
(2007).
Probing the role of the DXDD motif in Class II diterpene cyclases.
|
| |
Chembiochem,
8,
869-874.
|
 |
|
|
|
|
 |
C.Dürr,
H.J.Schnell,
A.Luzhetskyy,
R.Murillo,
M.Weber,
K.Welzel,
A.Vente,
and
A.Bechthold
(2006).
Biosynthesis of the terpene phenalinolactone in Streptomyces sp. Tü6071: analysis of the gene cluster and generation of derivatives.
|
| |
Chem Biol,
13,
365-377.
|
 |
|
|
|
|
 |
H.R.Corradi,
A.V.Corrigall,
E.Boix,
C.G.Mohan,
E.D.Sturrock,
P.N.Meissner,
and
K.R.Acharya
(2006).
Crystal structure of protoporphyrinogen oxidase from Myxococcus xanthus and its complex with the inhibitor acifluorfen.
|
| |
J Biol Chem,
281,
38625-38633.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Wuerges,
G.Garau,
S.Geremia,
S.N.Fedosov,
T.E.Petersen,
and
L.Randaccio
(2006).
Structural basis for mammalian vitamin B12 transport by transcobalamin.
|
| |
Proc Natl Acad Sci U S A,
103,
4386-4391.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.W.Fowler,
and
P.V.Coveney
(2006).
A computational protocol for the integration of the monotopic protein prostaglandin H2 synthase into a phospholipid bilayer.
|
| |
Biophys J,
91,
401-410.
|
 |
|
|
|
|
 |
R.E.Summons,
A.S.Bradley,
L.L.Jahnke,
and
J.R.Waldbauer
(2006).
Steroids, triterpenoids and molecular oxygen.
|
| |
Philos Trans R Soc Lond B Biol Sci,
361,
951-968.
|
 |
|
|
|
|
 |
S.P.Matsuda,
W.K.Wilson,
and
Q.Xiong
(2006).
Mechanistic insights into triterpene synthesis from quantum mechanical calculations. Detection of systematic errors in B3LYP cyclization energies.
|
| |
Org Biomol Chem,
4,
530-543.
|
 |
|
|
|
|
 |
K.U.Wendt
(2005).
Enzyme mechanisms for triterpene cyclization: new pieces of the puzzle.
|
| |
Angew Chem Int Ed Engl,
44,
3966-3971.
|
 |
|
|
|
|
 |
L.A.Wessjohann,
E.Ruijter,
D.Garcia-Rivera,
and
W.Brandt
(2005).
What can a chemist learn from nature's macrocycles?--a brief, conceptual view.
|
| |
Mol Divers,
9,
171-186.
|
 |
|
|
|
|
 |
M.Ceruti,
G.Balliano,
F.Rocco,
A.Lenhart,
G.E.Schulz,
F.Castelli,
and
P.Milla
(2005).
Synthesis and biological activity of new iodoacetamide derivatives on mutants of squalene-hopene cyclase.
|
| |
Lipids,
40,
729-735.
|
 |
|
|
|
|
 |
M.K.McKinney,
and
B.F.Cravatt
(2005).
Structure and function of fatty acid amide hydrolase.
|
| |
Annu Rev Biochem,
74,
411-432.
|
 |
|
|
|
|
 |
R.A.Yoder,
and
J.N.Johnston
(2005).
A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids.
|
| |
Chem Rev,
105,
4730-4756.
|
 |
|
|
|
|
 |
S.Oliaro-Bosso,
T.Schulz-Gasch,
G.Balliano,
and
F.Viola
(2005).
Access of the substrate to the active site of yeast oxidosqualene cyclase: an inhibition and site-directed mutagenesis approach.
|
| |
Chembiochem,
6,
2221-2228.
|
 |
|
|
|
|
 |
T.Abe,
and
T.Hoshino
(2005).
Enzymatic cyclizations of squalene analogs with threo- and erythro-diols at the 6,7- or 10,11-positions by recombinant squalene cyclase. Trapping of carbocation intermediates and mechanistic insights into the product and substrate specificities.
|
| |
Org Biomol Chem,
3,
3127-3139.
|
 |
|
|
|
|
 |
A.Goeke,
D.Mertl,
and
G.Brunner
(2004).
A novel approach to prezizaane sesquiterpenes.
|
| |
Chem Biodivers,
1,
1949-1956.
|
 |
|
|
|
|
 |
D.J.Reinert,
G.Balliano,
and
G.E.Schulz
(2004).
Conversion of squalene to the pentacarbocyclic hopene.
|
| |
Chem Biol,
11,
121-126.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.M.Cho,
A.Okada,
H.Kenmoku,
K.Otomo,
T.Toyomasu,
W.Mitsuhashi,
T.Sassa,
A.Yajima,
G.Yabuta,
K.Mori,
H.Oikawa,
H.Toshima,
N.Shibuya,
H.Nojiri,
T.Omori,
M.Nishiyama,
and
H.Yamane
(2004).
Molecular cloning and characterization of a cDNA encoding ent-cassa-12,15-diene synthase, a putative diterpenoid phytoalexin biosynthetic enzyme, from suspension-cultured rice cells treated with a chitin elicitor.
|
| |
Plant J,
37,
1-8.
|
 |
|
|
|
|
 |
G.Cravotto,
G.Balliano,
S.Tagliapietra,
S.Oliaro-Bosso,
and
G.M.Nano
(2004).
Novel squalene-hopene cyclase inhibitors derived from hydroxycoumarins and hydroxyacetophenones.
|
| |
Chem Pharm Bull (Tokyo),
52,
1171-1174.
|
 |
|
|
|
|
 |
H.Luo,
A.E.Yousef,
and
H.H.Wang
(2004).
A real-time polymerase chain reaction-based method for rapid and specific detection of spoilage Alicyclobacillus spp. in apple juice.
|
| |
Lett Appl Microbiol,
39,
376-382.
|
 |
|
|
|
|
 |
K.Poralla
(2004).
Profound insights into squalene cyclization.
|
| |
Chem Biol,
11,
12-14.
|
 |
|
|
|
|
 |
N.V.Chandrasekharan,
and
D.L.Simmons
(2004).
The cyclooxygenases.
|
| |
Genome Biol,
5,
241.
|
 |
|
|
|
|
 |
P.Benveniste
(2004).
Biosynthesis and accumulation of sterols.
|
| |
Annu Rev Plant Biol,
55,
429-457.
|
 |
|
|
|
|
 |
R.Thoma,
T.Schulz-Gasch,
B.D'Arcy,
J.Benz,
J.Aebi,
H.Dehmlow,
M.Hennig,
M.Stihle,
and
A.Ruf
(2004).
Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase.
|
| |
Nature,
432,
118-122.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Lodeiro,
M.J.Segura,
M.Stahl,
T.Schulz-Gasch,
and
S.P.Matsuda
(2004).
Oxidosqualene cyclase second-sphere residues profoundly influence the product profile.
|
| |
Chembiochem,
5,
1581-1585.
|
 |
|
|
|
|
 |
S.Oliaro-Bosso,
F.Viola,
S.Matsuda,
G.Cravotto,
S.Tagliapietra,
and
G.Balliano
(2004).
Umbelliferone aminoalkyl derivatives as inhibitors of oxidosqualene cyclases from Saccharomyces cerevisiae, Trypanosoma cruzi, and Pneumocystis carinii.
|
| |
Lipids,
39,
1007-1012.
|
 |
|
|
|
|
 |
T.Hoshino,
K.Shimizu,
and
T.Sato
(2004).
Deletion of the Gly600 residue of Alicyclobacillus acidocaldarius squalene cyclase alters the substrate specificity into that of the eukaryotic-type cyclase specific to (3S)-2,3-oxidosqualene.
|
| |
Angew Chem Int Ed Engl,
43,
6700-6703.
|
 |
|
|
|
|
 |
T.Sato,
M.Kouda,
and
T.Hoshino
(2004).
Site-directed mutagenesis experiments on the putative deprotonation site of squalene-hopene cyclase from Alicyclobacillus acidocaldarius.
|
| |
Biosci Biotechnol Biochem,
68,
728-738.
|
 |
|
|
|
|
 |
D.A.Berthold,
and
P.Stenmark
(2003).
Membrane-bound diiron carboxylate proteins.
|
| |
Annu Rev Plant Biol,
54,
497-517.
|
 |
|
|
|
|
 |
F.Rocco,
S.O.Bosso,
F.Viola,
P.Milla,
G.Roma,
G.Grossi,
and
M.Ceruti
(2003).
Conjugated methyl sulfide and phenyl sulfide derivatives of oxidosqualene as inhibitors of oxidosqualene and squalene-hopene cyclases.
|
| |
Lipids,
38,
201-207.
|
 |
|
|
|
|
 |
J.Wallace,
F.Harris,
and
D.A.Phoenix
(2003).
A statistical investigation of amphiphilic properties of C-terminally anchored peptidases.
|
| |
Eur Biophys J,
32,
589-598.
|
 |
|
|
|
|
 |
S.Maurer-Stroh,
S.Washietl,
and
F.Eisenhaber
(2003).
Protein prenyltransferases.
|
| |
Genome Biol,
4,
212.
|
 |
|
|
|
|
 |
D.A.Whittington,
M.L.Wise,
M.Urbansky,
R.M.Coates,
R.B.Croteau,
and
D.W.Christianson
(2002).
Bornyl diphosphate synthase: structure and strategy for carbocation manipulation by a terpenoid cyclase.
|
| |
Proc Natl Acad Sci U S A,
99,
15375-15380.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Ishibashi,
K.Ishihara,
and
H.Yamamoto
(2002).
Chiral proton donor reagents: tin tetrachloride--coordinated optically active binaphthol derivatives.
|
| |
Chem Rec,
2,
177-188.
|
 |
|
|
|
|
 |
J.Binet,
D.Thomas,
A.Benmbarek,
F.D.de,
and
P.Renaut
(2002).
Structure activity relationships of new inhibitors of mammalian 2,3-oxidosqualene cyclase designed from isoquinoline derivatives.
|
| |
Chem Pharm Bull (Tokyo),
50,
316-329.
|
 |
|
|
|
|
 |
P.H.Liang,
T.P.Ko,
and
A.H.Wang
(2002).
Structure, mechanism and function of prenyltransferases.
|
| |
Eur J Biochem,
269,
3339-3354.
|
 |
|
|
|
|
 |
P.Milla,
A.Lenhart,
G.Grosa,
F.Viola,
W.A.Weihofen,
G.E.Schulz,
and
G.Balliano
(2002).
Thiol-modifying inhibitors for understanding squalene cyclase function.
|
| |
Eur J Biochem,
269,
2108-2116.
|
 |
|
|
|
|
 |
P.Milla,
F.Viola,
S.Oliaro Bosso,
F.Rocco,
L.Cattel,
B.M.Joubert,
R.J.LeClair,
S.P.Matsuda,
and
G.Balliano
(2002).
Subcellular localization of oxidosqualene cyclases from Arabidopsis thaliana, Trypanosoma cruzi, and Pneumocystis carinii expressed in yeast.
|
| |
Lipids,
37,
1171-1176.
|
 |
|
|
|
|
 |
T.K.Wu,
and
J.H.Griffin
(2002).
Conversion of a plant oxidosqualene-cycloartenol synthase to an oxidosqualene-lanosterol cyclase by random mutagenesis.
|
| |
Biochemistry,
41,
8238-8244.
|
 |
|
|
|
|
 |
T.Sato,
S.Sasahara,
T.Yamakami,
and
T.Hoshino
(2002).
Functional analyses of Tyr420 and Leu607 of Alicyclobacillus acidocaldarius squalene-hopene cyclase. Neoachillapentaene, a novel triterpene with the 1,5,6-trimethylcyclohexene moiety produced through folding of the constrained boat structure.
|
| |
Biosci Biotechnol Biochem,
66,
1660-1670.
|
 |
|
|
|
|
 |
M.Ceruti,
G.Balliano,
F.Rocco,
P.Milla,
S.Arpicco,
L.Cattel,
and
F.Viola
(2001).
Vinyl sulfide derivatives of truncated oxidosqualene as selective inhibitors of oxidosqualene and squalene-hopene cyclases.
|
| |
Lipids,
36,
629-636.
|
 |
|
|
|
|
 |
M.Fujihashi,
Y.W.Zhang,
Y.Higuchi,
X.Y.Li,
T.Koyama,
and
K.Miki
(2001).
Crystal structure of cis-prenyl chain elongating enzyme, undecaprenyl diphosphate synthase.
|
| |
Proc Natl Acad Sci U S A,
98,
4337-4342.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.C.Trapp,
and
R.B.Croteau
(2001).
Genomic organization of plant terpene synthases and molecular evolutionary implications.
|
| |
Genetics,
158,
811-832.
|
 |
|
|
|
|
 |
T.Dairi,
Y.Hamano,
T.Kuzuyama,
N.Itoh,
K.Furihata,
and
H.Seto
(2001).
Eubacterial diterpene cyclase genes essential for production of the isoprenoid antibiotic terpentecin.
|
| |
J Bacteriol,
183,
6085-6094.
|
 |
|
|
|
|
 |
T.Sato,
and
T.Hoshino
(2001).
Catalytic function of the residues of phenylalanine and tyrosine conserved in squalene-hopene cyclases.
|
| |
Biosci Biotechnol Biochem,
65,
2233-2242.
|
 |
|
|
|
|
 |
T.Soderberg,
and
C.D.Poulter
(2001).
Escherichia coli dimethylallyl diphosphate:tRNA dimethylallyltransferase: site-directed mutagenesis of highly conserved residues.
|
| |
Biochemistry,
40,
1734-1740.
|
 |
|
|
|
|
 |
V.Durbecq,
G.Sainz,
Y.Oudjama,
B.Clantin,
C.Bompard-Gilles,
C.Tricot,
J.Caillet,
V.Stalon,
L.Droogmans,
and
V.Villeret
(2001).
Crystal structure of isopentenyl diphosphate:dimethylallyl diphosphate isomerase.
|
| |
EMBO J,
20,
1530-1537.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.A.Berthold,
M.E.Andersson,
and
P.Nordlund
(2000).
New insight into the structure and function of the alternative oxidase.
|
| |
Biochim Biophys Acta,
1460,
241-254.
|
 |
|
|
|
|
 |
D.E.Cane,
and
N.Ke
(2000).
Epicubenol synthase. Origin of the oxygen atom of a bacterial sesquiterpene alcohol.
|
| |
Bioorg Med Chem Lett,
10,
105-107.
|
 |
|
|
|
|
 |
F.Viola,
M.Ceruti,
L.Cattel,
P.Milla,
K.Poralla,
and
G.Balliano
(2000).
Rationally designed inhibitors as tools for comparing the mechanism of squalene-hopene cyclase with oxidosqualene cyclase.
|
| |
Lipids,
35,
297-303.
|
 |
|
|
|
|
 |
H.Zhang,
M.C.Seabra,
and
J.Deisenhofer
(2000).
Crystal structure of Rab geranylgeranyltransferase at 2.0 A resolution.
|
| |
Structure,
8,
241-251.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.L.Popot,
and
D.M.Engelman
(2000).
Helical membrane protein folding, stability, and evolution.
|
| |
Annu Rev Biochem,
69,
881-922.
|
 |
|
|
|
|
 |
K.U.Wendt,
G.E.Schulz,
E.J.Corey,
and
D.R.Liu
(2000).
Enzyme Mechanisms for Polycyclic Triterpene Formation.
|
| |
Angew Chem Int Ed Engl,
39,
2812-2833.
|
 |
|
|
|
|
 |
M.M.Meyer,
M.J.Segura,
W.K.Wilson,
and
S.P.Matsuda
(2000).
Oxidosqualene Cyclase Residues that Promote Formation of Cycloartenol, Lanosterol, and Parkeol We are grateful to Bridget M. Joubert for advice regarding mutagenesis. We thank Elizabeth A. Hart for an authentic parkeol standard, and for chromatographic and spectroscopic information. This research was funded by the National Institutes of Health (grant no. AI 41598) and the Robert A. Welch Foundation (grant no. C-1323). M.M.M. was an American Society of Pharmacognosy Undergraduate Fellow. M.J.R.S. was a Robert A. Welch Fellow and was supported by an NIH Biotechnology Training Grant (grant no. T32 GM08362).
|
| |
Angew Chem Int Ed Engl,
39,
4090-4092.
|
 |
|
|
|
|
 |
R.J.Peters,
J.E.Flory,
R.Jetter,
M.M.Ravn,
H.J.Lee,
R.M.Coates,
and
R.B.Croteau
(2000).
Abietadiene synthase from grand fir (Abies grandis): characterization and mechanism of action of the "pseudomature" recombinant enzyme.
|
| |
Biochemistry,
39,
15592-15602.
|
 |
|
|
|
|
 |
S.M.Godzina,
M.A.Lovato,
M.M.Meyer,
K.A.Foster,
W.K.Wilson,
W.Gu,
E.L.de Hostos,
and
S.P.Matsuda
(2000).
Cloning and characterization of the Dictyostelium discoideum cycloartenol synthase cDNA.
|
| |
Lipids,
35,
249-255.
|
 |
|
|
|
|
 |
T.Dang,
and
G.D.Prestwich
(2000).
Site-directed mutagenesis of squalene-hopene cyclase: altered substrate specificity and product distribution.
|
| |
Chem Biol,
7,
643-649.
|
 |
|
|
|
|
 |
A.S.Richman,
M.Gijzen,
A.N.Starratt,
Z.Yang,
and
J.E.Brandle
(1999).
Diterpene synthesis in Stevia rebaudiana: recruitment and up-regulation of key enzymes from the gibberellin biosynthetic pathway.
|
| |
Plant J,
19,
411-421.
|
 |
|
|
|
|
 |
C.M.Apfel,
B.Takács,
M.Fountoulakis,
M.Stieger,
and
W.Keck
(1999).
Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: cloning, expression, and characterization of the essential uppS gene.
|
| |
J Bacteriol,
181,
483-492.
|
 |
|
|
|
|
 |
C.W.Wang,
M.K.Oh,
and
J.C.Liao
(1999).
Engineered isoprenoid pathway enhances astaxanthin production in Escherichia coli.
|
| |
Biotechnol Bioeng,
62,
235-241.
|
 |
|
|
|
|
 |
F.F.Moebius,
K.E.Soellner,
B.Fiechtner,
C.W.Huck,
G.Bonn,
and
H.Glossmann
(1999).
Histidine77, glutamic acid81, glutamic acid123, threonine126, asparagine194, and tryptophan197 of the human emopamil binding protein are required for in vivo sterol delta 8-delta 7 isomerization.
|
| |
Biochemistry,
38,
1119-1127.
|
 |
|
|
|
|
 |
K.Wang,
and
S.Ohnuma
(1999).
Chain-length determination mechanism of isoprenyl diphosphate synthases and implications for molecular evolution.
|
| |
Trends Biochem Sci,
24,
445-451.
|
 |
|
|
|
|
 |
M.Fujihashi,
N.Shimizu,
Y.W.Zhang,
T.Koyama,
and
K.Miki
(1999).
Crystallization and preliminary X-ray diffraction studies of undecaprenyl diphosphate synthase from Micrococcus luteus B-P 26.
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
1606-1607.
|
 |
|
|
|
|
 |
T.Dang,
I.Abe,
Y.F.Zheng,
and
G.D.Prestwich
(1999).
The binding site for an inhibitor of squalene:hopene cyclase determined using photoaffinity labeling and molecular modeling.
|
| |
Chem Biol,
6,
333-341.
|
 |
|
|
|
|
 |
T.Hoshino,
M.Kouda,
T.Abe,
and
S.Ohashi
(1999).
New cyclization mechanism for squalene: a ring-expansion step for the five-membered C-ring intermediate in hopene biosynthesis.
|
| |
Biosci Biotechnol Biochem,
63,
2038-2041.
|
 |
|
|
|
|
 |
T.Sato,
and
T.Hoshino
(1999).
Kinetic studies on the function of all the conserved tryptophans involved inside and outside the QW motifs of squalene-hopene cyclase: stabilizing effect of the protein structure against thermal denaturation.
|
| |
Biosci Biotechnol Biochem,
63,
1171-1180.
|
 |
|
|
|
|
 |
T.Sato,
and
T.Hoshino
(1999).
Functional analysis of the DXDDTA motif in squalene-hopene cyclase by site-directed mutagenesis experiments: initiation site of the polycyclization reaction and stabilization site of the carbocation intermediate of the initially cyclized A-ring.
|
| |
Biosci Biotechnol Biochem,
63,
2189-2198.
|
 |
|
|
|
|
 |
C.A.Lesburg,
J.M.Caruthers,
C.M.Paschall,
and
D.W.Christianson
(1998).
Managing and manipulating carbocations in biology: terpenoid cyclase structure and mechanism.
|
| |
Curr Opin Struct Biol,
8,
695-703.
|
 |
|
|
|
|
 |
I.Abe,
Y.F.Zheng,
and
G.D.Prestwich
(1998).
Photoaffinity labeling of oxidosqualene cyclase and squalene cyclase by a benzophenone-containing inhibitor.
|
| |
Biochemistry,
37,
5779-5784.
|
 |
|
|
|
|
 |
J.Bohlmann,
G.Meyer-Gauen,
and
R.Croteau
(1998).
Plant terpenoid synthases: molecular biology and phylogenetic analysis.
|
| |
Proc Natl Acad Sci U S A,
95,
4126-4133.
|
 |
|
|
|
|
 |
K.U.Wendt,
and
G.E.Schulz
(1998).
Isoprenoid biosynthesis: manifold chemistry catalyzed by similar enzymes.
|
| |
Structure,
6,
127-133.
|
 |
|
|
|
|
 |
M.P.Patricelli,
H.A.Lashuel,
D.K.Giang,
J.W.Kelly,
and
B.F.Cravatt
(1998).
Comparative characterization of a wild type and transmembrane domain-deleted fatty acid amide hydrolase: identification of the transmembrane domain as a site for oligomerization.
|
| |
Biochemistry,
37,
15177-15187.
|
 |
|
|
|
|
 |
R.M.Garavito
(1998).
Membrane protein structures: the known world expands.
|
| |
Curr Opin Biotechnol,
9,
344-349.
|
 |
|
|
|
|
 |
S.Arnould,
and
J.M.Camadro
(1998).
The domain structure of protoporphyrinogen oxidase, the molecular target of diphenyl ether-type herbicides.
|
| |
Proc Natl Acad Sci U S A,
95,
10553-10558.
|
 |
|
|
|
|
 |
T.Sato,
Y.Kanai,
and
T.Hoshino
(1998).
Overexpression of squalene-hopene cyclase by the pET vector in Escherichia coli and first identification of tryptophan and aspartic acid residues inside the QW motif as active sites.
|
| |
Biosci Biotechnol Biochem,
62,
407-411.
|
 |
|
|
|
|
 |
Y.F.Zheng,
I.Abe,
and
G.D.Prestwich
(1998).
Inhibition kinetics and affinity labeling of bacterial squalene:hopene cyclase by thia-substituted analogues of 2, 3-oxidosqualene.
|
| |
Biochemistry,
37,
5981-5987.
|
 |
|
|
|
|
 |
C.A.Townsend
(1997).
Structural studies of natural product biosynthetic proteins.
|
| |
Chem Biol,
4,
721-730.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

