 |
PDBsum entry 1d3h

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1d3h
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.3.5.2
- dihydroorotate dehydrogenase (quinone).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(S)-dihydroorotate + a quinone = orotate + a quinol
|
 |
 |
 |
 |
 |
(S)-dihydroorotate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 quinone
quinone
|
=
|
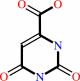 orotate
orotate
|
+
|
quinol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FMN
|
 |
 |
 |
 |
 |
FMN
Bound ligand (Het Group name =
FMN)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure Fold Des
8:25-33
(2000)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structures of human dihydroorotate dehydrogenase in complex with antiproliferative agents.
|
|
S.Liu,
E.A.Neidhardt,
T.H.Grossman,
T.Ocain,
J.Clardy.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
BACKGROUND: Dihydroorotate dehydrogenase (DHODH) catalyzes the fourth committed
step in the de novo biosynthesis of pyrimidines. As rapidly proliferating human
T cells have an exceptional requirement for de novo pyrimidine biosynthesis,
small molecule DHODH inhibitors constitute an attractive therapeutic approach to
autoimmune diseases, immunosuppression, and cancer. Neither the structure of
human DHODH nor any member of its family was known. RESULTS: The high-resolution
crystal structures of human DHODH in complex with two different inhibitors have
been solved. The initial set of phases was obtained using multiwavelength
anomalous diffraction phasing with selenomethionine-containing DHODH. The
structures have been refined to crystallographic R factors of 16.8% and 16.2% at
resolutions of 1. 6 A and 1.8 A for inhibitors related to brequinar and
leflunomide, respectively. CONCLUSIONS: Human DHODH has two domains: an
alpha/beta-barrel domain containing the active site and an alpha-helical domain
that forms the opening of a tunnel leading to the active site. Both inhibitors
share a common binding site in this tunnel, and differences in the binding
region govern drug sensitivity or resistance. The active site of human DHODH is
generally similar to that of the previously reported bacterial active site. The
greatest differences are that the catalytic base removing the proton from
dihydroorotate is a serine rather than a cysteine, and that packing of the
flavin mononucleotide in its binding site is tighter.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |
Figure 5.
Figure 5. Binding of A771726 in the hydrophobic channel of
human DHODH. (a) Stereoview of the binding interactions of
A771726. Ligands are shown in ball-and-stick representation, and
residues interacting with A771726 are represented by thin
sticks. Hydrogen bonds are shown as dashed black lines. (b) A
2F[o]-F[c ]electron-density map of A771726, FMN and orotate
contoured at 1.2s (1.8 ┼). Ligands and selected DHODH residues
in the vicinity are shown in ball-and-stick representation. This
figure was prepared with MOLSCRIPT [34].
|
 |
|
|

|
| |
The above figure is
reprinted
by permission from Cell Press:
Structure Fold Des
(2000,
8,
25-33)
copyright 2000.
|
|
| |
Figure was
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.H.Lu,
J.W.Wu,
H.L.Liu,
J.H.Zhao,
K.T.Liu,
C.K.Chuang,
H.Y.Lin,
W.B.Tsai,
and
Y.Ho
(2011).
The discovery of potential acetylcholinesterase inhibitors: a combination of pharmacophore modeling, virtual screening, and molecular docking studies.
|
| |
J Biomed Sci,
18,
8.
|
 |
|
|
|
|
 |
C.Desler,
A.Lykke,
and
L.J.Rasmussen
(2010).
The effect of mitochondrial dysfunction on cytosolic nucleotide metabolism.
|
| |
J Nucleic Acids,
2010,
0.
|
 |
|
|
|
|
 |
G.Lenaz,
and
M.L.Genova
(2010).
Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject.
|
| |
Antioxid Redox Signal,
12,
961.
|
 |
|
|
|
|
 |
I.Fritzson,
B.Svensson,
S.Al-Karadaghi,
B.Walse,
U.Wellmar,
U.J.Nilsson,
D.da Graça Thrige,
and
S.Jönsson
(2010).
Inhibition of human DHODH by 4-hydroxycoumarins, fenamic acids, and N-(alkylcarbonyl)anthranilic acids identified by structure-guided fragment selection.
|
| |
ChemMedChem,
5,
608-617.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Möbius,
R.Arias-Cartin,
D.Breckau,
A.L.Hännig,
K.Riedmann,
R.Biedendieck,
S.Schröder,
D.Becher,
A.Magalon,
J.Moser,
M.Jahn,
and
D.Jahn
(2010).
Heme biosynthesis is coupled to electron transport chains for energy generation.
|
| |
Proc Natl Acad Sci U S A,
107,
10436-10441.
|
 |
|
|
|
|
 |
M.A.Phillips,
and
P.K.Rathod
(2010).
Plasmodium dihydroorotate dehydrogenase: a promising target for novel anti-malarial chemotherapy.
|
| |
Infect Disord Drug Targets,
10,
226-239.
|
 |
|
|
|
|
 |
M.Qing,
G.Zou,
Q.Y.Wang,
H.Y.Xu,
H.Dong,
Z.Yuan,
and
P.Y.Shi
(2010).
Characterization of dengue virus resistance to brequinar in cell culture.
|
| |
Antimicrob Agents Chemother,
54,
3686-3695.
|
 |
|
|
|
|
 |
O.P.Kulkarni,
S.G.Sayyed,
C.Kantner,
M.Ryu,
M.Schnurr,
M.Sárdy,
J.Leban,
R.Jankowsky,
A.Ammendola,
R.Doblhofer,
and
H.J.Anders
(2010).
4SC-101, a novel small molecule dihydroorotate dehydrogenase inhibitor, suppresses systemic lupus erythematosus in MRL-(Fas)lpr mice.
|
| |
Am J Pathol,
176,
2840-2847.
|
 |
|
|
|
|
 |
P.R.Rich,
and
A.Maréchal
(2010).
The mitochondrial respiratory chain.
|
| |
Essays Biochem,
47,
1.
|
 |
|
|
|
|
 |
S.Teschner,
and
V.Burst
(2010).
Leflunomide: a drug with a potential beyond rheumatology.
|
| |
Immunotherapy,
2,
637-650.
|
 |
|
|
|
|
 |
G.Schneider,
M.Hartenfeller,
M.Reutlinger,
Y.Tanrikulu,
E.Proschak,
and
P.Schneider
(2009).
Voyages to the (un)known: adaptive design of bioactive compounds.
|
| |
Trends Biotechnol,
27,
18-26.
|
 |
|
|
|
|
 |
R.Gujjar,
A.Marwaha,
F.El Mazouni,
J.White,
K.L.White,
S.Creason,
D.M.Shackleford,
J.Baldwin,
W.N.Charman,
F.S.Buckner,
S.Charman,
P.K.Rathod,
and
M.A.Phillips
(2009).
Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice.
|
| |
J Med Chem,
52,
1864-1872.
|
 |
|
|
|
|
 |
R.L.Fagan,
and
B.A.Palfey
(2009).
Roles in binding and chemistry for conserved active site residues in the class 2 dihydroorotate dehydrogenase from Escherichia coli.
|
| |
Biochemistry,
48,
7169-7178.
|
 |
|
|
|
|
 |
R.L.Kow,
J.R.Whicher,
C.A.McDonald,
B.A.Palfey,
and
R.L.Fagan
(2009).
Disruption of the proton relay network in the class 2 dihydroorotate dehydrogenase from Escherichia coli.
|
| |
Biochemistry,
48,
9801-9809.
|
 |
|
|
|
|
 |
X.Deng,
R.Gujjar,
F.El Mazouni,
W.Kaminsky,
N.A.Malmquist,
E.J.Goldsmith,
P.K.Rathod,
and
M.A.Phillips
(2009).
Structural plasticity of malaria dihydroorotate dehydrogenase allows selective binding of diverse chemical scaffolds.
|
| |
J Biol Chem,
284,
26999-27009.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.A.Phillips,
R.Gujjar,
N.A.Malmquist,
J.White,
F.El Mazouni,
J.Baldwin,
and
P.K.Rathod
(2008).
Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum.
|
| |
J Med Chem,
51,
3649-3653.
|
 |
|
|
|
|
 |
N.A.Malmquist,
R.Gujjar,
P.K.Rathod,
and
M.A.Phillips
(2008).
Analysis of flavin oxidation and electron-transfer inhibition in Plasmodium falciparum dihydroorotate dehydrogenase.
|
| |
Biochemistry,
47,
2466-2475.
|
 |
|
|
|
|
 |
S.G.Couto,
M.C.Nonato,
and
A.J.Costa-Filho
(2008).
Defects in vesicle core induced by escherichia coli dihydroorotate dehydrogenase.
|
| |
Biophys J,
94,
1746-1753.
|
 |
|
|
|
|
 |
T.L.Arakaki,
F.S.Buckner,
J.R.Gillespie,
N.A.Malmquist,
M.A.Phillips,
O.Kalyuzhniy,
J.R.Luft,
G.T.Detitta,
C.L.Verlinde,
W.C.Van Voorhis,
W.G.Hol,
and
E.A.Merritt
(2008).
Characterization of Trypanosoma brucei dihydroorotate dehydrogenase as a possible drug target; structural, kinetic and RNAi studies.
|
| |
Mol Microbiol,
68,
37-50.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.Patel,
M.Booker,
M.Kramer,
L.Ross,
C.A.Celatka,
L.M.Kennedy,
J.D.Dvorin,
M.T.Duraisingh,
P.Sliz,
D.F.Wirth,
and
J.Clardy
(2008).
Identification and Characterization of Small Molecule Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase.
|
| |
J Biol Chem,
283,
35078-35085.
|
 |
|
|
|
|
 |
C.H.Tung,
J.W.Huang,
and
J.M.Yang
(2007).
Kappa-alpha plot derived structural alphabet and BLOSUM-like substitution matrix for rapid search of protein structure database.
|
| |
Genome Biol,
8,
R31.
|
 |
|
|
|
|
 |
E.Zameitat,
A.J.Pierik,
K.Zocher,
and
M.Löffler
(2007).
Dihydroorotate dehydrogenase from Saccharomyces cerevisiae: spectroscopic investigations with the recombinant enzyme throw light on catalytic properties and metabolism of fumarate analogues.
|
| |
FEMS Yeast Res,
7,
897-904.
|
 |
|
|
|
|
 |
E.Zameitat,
G.Freymark,
C.D.Dietz,
M.Löffler,
and
M.Bölker
(2007).
Functional expression of human dihydroorotate dehydrogenase (DHODH) in pyr4 mutants of ustilago maydis allows target validation of DHODH inhibitors in vivo.
|
| |
Appl Environ Microbiol,
73,
3371-3379.
|
 |
|
|
|
|
 |
N.A.Malmquist,
J.Baldwin,
and
M.A.Phillips
(2007).
Detergent-dependent kinetics of truncated Plasmodium falciparum dihydroorotate dehydrogenase.
|
| |
J Biol Chem,
282,
12678-12686.
|
 |
|
|
|
|
 |
D.E.Hurt,
J.Widom,
and
J.Clardy
(2006).
Structure of Plasmodium falciparum dihydroorotate dehydrogenase with a bound inhibitor.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
312-323.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.Zameitat,
Z.Gojkovi─ç,
W.Knecht,
J.Piskur,
and
M.Löffler
(2006).
Biochemical characterization of recombinant dihydroorotate dehydrogenase from the opportunistic pathogenic yeast Candida albicans.
|
| |
FEBS J,
273,
3183-3191.
|
 |
|
|
|
|
 |
T.Herz,
K.Wolf,
J.Kraus,
and
B.Kramer
(2006).
4SCan/vADME: intelligent library screening as a shortcut from hits to lead compounds.
|
| |
Expert Opin Drug Metab Toxicol,
2,
471-484.
|
 |
|
|
|
|
 |
J.Baldwin,
C.H.Michnoff,
N.A.Malmquist,
J.White,
M.G.Roth,
P.K.Rathod,
and
M.A.Phillips
(2005).
High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase.
|
| |
J Biol Chem,
280,
21847-21853.
|
 |
|
|
|
|
 |
D.R.Evans,
and
H.I.Guy
(2004).
Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway.
|
| |
J Biol Chem,
279,
33035-33038.
|
 |
|
|
|
|
 |
M.Hansen,
J.Le Nours,
E.Johansson,
T.Antal,
A.Ullrich,
M.Löffler,
and
S.Larsen
(2004).
Inhibitor binding in a class 2 dihydroorotate dehydrogenase causes variations in the membrane-associated N-terminal domain.
|
| |
Protein Sci,
13,
1031-1042.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Puranik,
S.B.Nielsen,
H.Youn,
A.N.Hvitved,
J.L.Bourassa,
M.A.Case,
C.Tengroth,
G.Balakrishnan,
M.V.Thorsteinsson,
J.T.Groves,
G.L.McLendon,
G.P.Roberts,
J.S.Olson,
and
T.G.Spiro
(2004).
Dynamics of carbon monoxide binding to CooA.
|
| |
J Biol Chem,
279,
21096-21108.
|
 |
|
|
|
|
 |
S.Nørager,
S.Arent,
O.Björnberg,
M.Ottosen,
L.Lo Leggio,
K.F.Jensen,
and
S.Larsen
(2003).
Lactococcus lactis dihydroorotate dehydrogenase A mutants reveal important facets of the enzymatic function.
|
| |
J Biol Chem,
278,
28812-28822.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.B.Ottosen,
O.Björnberg,
S.Nørager,
S.Larsen,
B.A.Palfey,
and
K.F.Jensen
(2002).
The dimeric dihydroorotate dehydrogenase A from Lactococcus lactis dissociates reversibly into inactive monomers.
|
| |
Protein Sci,
11,
2575-2583.
|
 |
|
|
|
|
 |
S.Nørager,
K.F.Jensen,
O.Björnberg,
and
S.Larsen
(2002).
E. coli dihydroorotate dehydrogenase reveals structural and functional distinctions between different classes of dihydroorotate dehydrogenases.
|
| |
Structure,
10,
1211-1223.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Ullrich,
W.Knecht,
M.Fries,
and
M.Löffler
(2001).
Recombinant expression of N-terminal truncated mutants of the membrane bound mouse, rat and human flavoenzyme dihydroorotate dehydrogenase. A versatile tool to rate inhibitor effects?
|
| |
Eur J Biochem,
268,
1861-1868.
|
 |
|
|
|
|
 |
B.A.Palfey,
O.Björnberg,
and
K.F.Jensen
(2001).
Insight into the chemistry of flavin reduction and oxidation in Escherichia coli dihydroorotate dehydrogenase obtained by rapid reaction studies.
|
| |
Biochemistry,
40,
4381-4390.
|
 |
|
|
|
|
 |
P.Rowland,
S.Nørager,
K.F.Jensen,
and
S.Larsen
(2000).
Structure of dihydroorotate dehydrogenase B: electron transfer between two flavin groups bridged by an iron-sulphur cluster.
|
| |
Structure,
8,
1227-1238.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

