What are protein domains?
Domains are distinct functional and/or structural units in a protein. Usually they are responsible for a particular function or interaction, contributing to the overall role of a protein. Domains may exist in a variety of biological contexts, where similar domains can be found in proteins with different functions.
For example, Src homology 3 (SH3) domains are small domains of around 50 amino acid residues that are involved in protein-protein interactions. SH3 domains have a characteristic 3D structure (Figure 4). They occur in a diverse range of proteins with different functions, including adaptor proteins, phosphatidylinositol 3-kinases, phospholipases and myosins.

An example of a protein that contains multiple SH3 domains is the cytoplasmic protein Nck. Nck belongs to the adaptor family of proteins and it is involved in transducing signals from growth factor receptor tyrosine kinases to downstream signal recipients. The domain composition of Nck is illustrated in Figure 5.

As we have just seen with Nck, proteins can be composed of multiple domains. Often the individual domains have specific functions, such as binding a particular molecule or catalysing a given reaction, and together these contribute to the overall role of the protein (see, for example, the domain composition of the enzyme phospholipase D1 in Figure 6 below).
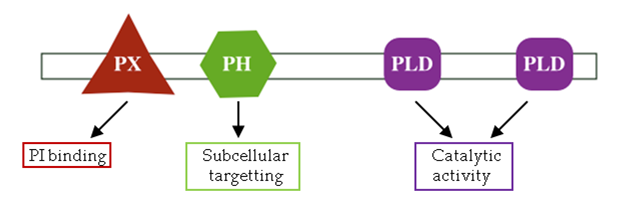
The protein contains a PX (phox) domain that is involved in binding phosphatidylinositol, a PH (pleckstrin homology) domain that has a role in targeting the enzyme to particular locations within the cell, and two PLD (phospholipase D) domains responsible for the protein’s catalytic activity.