|
Figure 4.
Figure 4 Binding of CoA to the putative active site of YfdW. A
CoA omit map contoured at 2  is
shown in magenta. CoA is depicted in blue in an all-atom
representation and the water molecules are shown as red spheres.
All CoA interactions are within the large domain. The adenine
moiety is positioned between the side chains of Arg38, Thr74,
Phe97 and Met105. There are hydrogen-bonding interactions
between adenine N6 and the carbonyl O atom of Leu72 and a
water-mediated hydrogen bond between adenine N7 and the carbonyl
O atom of Ile36. For the ribose moiety, O4' forms a hydrogen
bond to Arg38 NH1 and O2' forms a water-mediated hydrogen bond
to the carbonyl O atom of Ala101. The ribose phosphate moiety
forms hydrogen bonds to His98 N is
shown in magenta. CoA is depicted in blue in an all-atom
representation and the water molecules are shown as red spheres.
All CoA interactions are within the large domain. The adenine
moiety is positioned between the side chains of Arg38, Thr74,
Phe97 and Met105. There are hydrogen-bonding interactions
between adenine N6 and the carbonyl O atom of Leu72 and a
water-mediated hydrogen bond between adenine N7 and the carbonyl
O atom of Ile36. For the ribose moiety, O4' forms a hydrogen
bond to Arg38 NH1 and O2' forms a water-mediated hydrogen bond
to the carbonyl O atom of Ala101. The ribose phosphate moiety
forms hydrogen bonds to His98 N  2
through O9 and O7 and to Lys75 N 2
through O9 and O7 and to Lys75 N 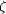 through
O8. The binding of the CoA pantetheine chain is stabilized by
van der Waals interactions with Met200 and several
hydrogen-bonding interactions. AO1 of the pyrophosphate moiety
hydrogen bonds to Arg38 N through
O8. The binding of the CoA pantetheine chain is stabilized by
van der Waals interactions with Met200 and several
hydrogen-bonding interactions. AO1 of the pyrophosphate moiety
hydrogen bonds to Arg38 N 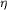 2,
AO4 to the hydroxyl of Tyr139 through two water molecules and
AO5 to Lys137 N 2,
AO4 to the hydroxyl of Tyr139 through two water molecules and
AO5 to Lys137 N 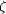 .
The pantetheine hydroxyl group hydrogen bonds to the amide of
His98 and the pantetheine amino PN4 interacts with the carbonyl
O atom of Ala138 and PN8 with the carbonyl O atom of Asn96. The
pantetheine carbonyl O atom PO5 interacts with Asn96 ND2, which
is stabilized by a hydrogen bond to Ser18 OG. The pantetheine
SH group PS1 hydrogen bonds to the amide of Asn17 and is also in
close contact with Asp169 OD2. The SH group also appears to be
stabilized by a helix dipole interaction with the N-terminus of .
The pantetheine hydroxyl group hydrogen bonds to the amide of
His98 and the pantetheine amino PN4 interacts with the carbonyl
O atom of Ala138 and PN8 with the carbonyl O atom of Asn96. The
pantetheine carbonyl O atom PO5 interacts with Asn96 ND2, which
is stabilized by a hydrogen bond to Ser18 OG. The pantetheine
SH group PS1 hydrogen bonds to the amide of Asn17 and is also in
close contact with Asp169 OD2. The SH group also appears to be
stabilized by a helix dipole interaction with the N-terminus of
 -helix
1. -helix
1.
|
