 |
PDBsum entry 2w0b

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2w0b
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.15.36
- sterol 14alpha-demethylase (ferredoxin).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 14alpha-methyl steroid + 6 reduced [2Fe-2S]-[ferredoxin] + 3 O2 + 5 H+ = a Delta14 steroid + formate + 6 oxidized [2Fe-2S]-[ferredoxin] + 4 H2O
|
 |
 |
 |
 |
 |
14alpha-methyl steroid
|
+
|
6
×
reduced [2Fe-2S]-[ferredoxin]
|
+
|
 3
×
O2
3
×
O2
|
+
|
5
×
H(+)
|
=
|
Delta(14) steroid
|
+
|
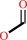 formate
formate
|
+
|
6
×
oxidized [2Fe-2S]-[ferredoxin]
|
+
|
4
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Plos Negl Trop Dis
3:e372
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Trypanosoma cruzi CYP51 inhibitor derived from a Mycobacterium tuberculosis screen hit.
|
|
C.K.Chen,
P.S.Doyle,
L.V.Yermalitskaya,
Z.B.Mackey,
K.K.Ang,
J.H.McKerrow,
L.M.Podust.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
BACKGROUND: The two front-line drugs for chronic Trypanosoma cruzi infections
are limited by adverse side-effects and declining efficacy. One potential new
target for Chagas' disease chemotherapy is sterol 14alpha-demethylase (CYP51), a
cytochrome P450 enzyme involved in biosynthesis of membrane sterols.
METHODOLOGY/PRINCIPAL FINDING: In a screening effort targeting Mycobacterium
tuberculosis CYP51 (CYP51(Mt)), we previously identified the
N-[4-pyridyl]-formamide moiety as a building block capable of delivering a
variety of chemotypes into the CYP51 active site. In that work, the binding
modes of several second generation compounds carrying this scaffold were
determined by high-resolution co-crystal structures with CYP51(Mt). Subsequent
assays against the CYP51 orthologue in T. cruzi, CYP51(Tc), demonstrated that
two of the compounds tested in the earlier effort bound tightly to this enzyme.
Both were tested in vitro for inhibitory effects against T. cruzi and the
related protozoan parasite Trypanosoma brucei, the causative agent of African
sleeping sickness. One of the compounds had potent, selective anti-T. cruzi
activity in infected mouse macrophages. Cure of treated host cells was confirmed
by prolonged incubation in the absence of the inhibiting compound.
Discrimination between T. cruzi and T. brucei CYP51 by the inhibitor was largely
based on the variability (phenylalanine versus isoleucine) of a single residue
at a critical position in the active site. CONCLUSIONS/SIGNIFICANCE:
CYP51(Mt)-based crystal structure analysis revealed that the functional groups
of the two tightly bound compounds are likely to occupy different spaces in the
CYP51 active site, suggesting the possibility of combining the beneficial
features of both inhibitors in a third generation of compounds to achieve more
potent and selective inhibition of CYP51(Tc).

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
P.S.Doyle,
C.K.Chen,
J.B.Johnston,
S.D.Hopkins,
S.S.Leung,
M.P.Jacobson,
J.C.Engel,
J.H.McKerrow,
and
L.M.Podust
(2010).
A nonazole CYP51 inhibitor cures Chagas' disease in a mouse model of acute infection.
|
| |
Antimicrob Agents Chemother,
54,
2480-2488.
|
 |
|
|
|
|
 |
T.C.Pochapsky,
S.Kazanis,
and
M.Dang
(2010).
Conformational plasticity and structure/function relationships in cytochromes P450.
|
| |
Antioxid Redox Signal,
13,
1273-1296.
|
 |
|
|
|
|
 |
L.M.Podust,
H.Ouellet,
J.P.von Kries,
and
P.R.de Montellano
(2009).
Interaction of Mycobacterium tuberculosis CYP130 with heterocyclic arylamines.
|
| |
J Biol Chem,
284,
25211-25219.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

