 |
PDBsum entry 2v1p

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.1.99.1
- tryptophanase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-tryptophan + H2O = indole + pyruvate + NH4+
|
 |
 |
 |
 |
 |
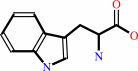 L-tryptophan
L-tryptophan
|
+
|
H2O
|
=
|
 indole
indole
|
+
|
 pyruvate
pyruvate
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
K(+); Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
K(+)
|
 Pyridoxal 5'-phosphate
Pyridoxal 5'-phosphate
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Bmc Struct Biol
9:65
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Conformational changes and loose packing promote E. coli Tryptophanase cold lability.
|
|
A.Kogan,
G.Y.Gdalevsky,
R.Cohen-Luria,
Y.Goldgur,
R.S.Phillips,
A.H.Parola,
O.Almog.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
BACKGROUND: Oligomeric enzymes can undergo a reversible loss of activity at low
temperatures. One such enzyme is tryptophanase (Trpase) from Escherichia coli.
Trpase is a pyridoxal phosphate (PLP)-dependent tetrameric enzyme with a Mw of
210 kD. PLP is covalently bound through an enamine bond to Lys270 at the active
site. The incubation of holo E. coli Trpases at 2 degrees C for 20 h results in
breaking this enamine bond and PLP release, as well as a reversible loss of
activity and dissociation into dimers. This sequence of events is termed cold
lability and its understanding bears relevance to protein stability and shelf
life. RESULTS: We studied the reversible cold lability of E. coli Trpase and its
Y74F, C298S and W330F mutants. In contrast to the holo E. coli Trpase all apo
forms of Trpase dissociated into dimers already at 25 degrees C and even further
upon cooling to 2 degrees C. The crystal structures of the two mutants, Y74F and
C298S in their apo form were determined at 1.9A resolution. These apo mutants
were found in an open conformation compared to the closed conformation found for
P. vulgaris in its holo form. This conformational change is further supported by
a high pressure study. CONCLUSION: We suggest that cold lability of E. coli
Trpases is primarily affected by PLP release. The enhanced loss of activity of
the three mutants is presumably due to the reduced size of the side chain of the
amino acids. This prevents the tight assembly of the active tetramer, making it
more susceptible to the cold driven changes in hydrophobic interactions which
facilitate PLP release. The hydrophobic interactions along the non catalytic
interface overshadow the effect of point mutations and may account for the
differences in the dissociation of E. coli Trpase to dimers and P. vulgaris
Trpase to monomers.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

