 |
PDBsum entry 2jku

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Crystal structure of the n-terminal region of the biotin acceptor domain of human propionyl-coa carboxylase
|
|
Structure:
|
 |
Propionyl-coa carboxylase alpha chain, mitochondrial. Chain: a. Fragment: biotin acceptor domain, residues 633-703. Synonym: propanoyl-coa\:carbon dioxide ligase subunit alpha. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Organelle: mitochondria. Expressed in: escherichia coli. Expression_system_taxid: 469008. Expression_system_variant: r3-prare2.
|
|
Resolution:
|
 |
|
1.50Å
|
R-factor:
|
0.149
|
R-free:
|
0.166
|
|
|
Authors:
|
 |
S.Healy,W.W.Yue,G.Kochan,E.S.Pilka,J.W.Murray,A.K.Roos, P.Filippakopoulos,F.Von Delft,C.Arrowsmith,M.Wikstrom,A.Edwards, C.Bountra,R.A.Gravel,U.Oppermann
|
|
Key ref:
|
 |
S.Healy
et al.
(2010).
Structural impact of human and Escherichia coli biotin carboxyl carrier proteins on biotin attachment.
Biochemistry,
49,
4687-4694.
PubMed id:
|
 |
|
Date:
|
 |
|
30-Aug-08
|
Release date:
|
09-Sep-08
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P05165
(PCCA_HUMAN) -
Propionyl-CoA carboxylase alpha chain, mitochondrial from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
728 a.a.
35 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.4.1.3
- propionyl-CoA carboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
propanoyl-CoA + hydrogencarbonate + ATP = (S)-methylmalonyl-CoA + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
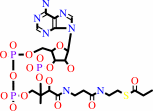 propanoyl-CoA
propanoyl-CoA
|
+
|
 hydrogencarbonate
hydrogencarbonate
|
+
|
 ATP
ATP
|
=
|
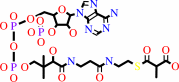 (S)-methylmalonyl-CoA
(S)-methylmalonyl-CoA
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Biotin
|
 |
 |
 |
 |
 |
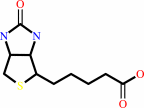 Biotin
Biotin
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
49:4687-4694
(2010)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural impact of human and Escherichia coli biotin carboxyl carrier proteins on biotin attachment.
|
|
S.Healy,
M.K.McDonald,
X.Wu,
W.W.Yue,
G.Kochan,
U.Oppermann,
R.A.Gravel.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Holocarboxylase synthetase (HCS, human) and BirA (Escherichia coli) are biotin
protein ligases that catalyze the ATP-dependent attachment of biotin to
apocarboxylases. Biotin attachment occurs on a highly conserved lysine residue
within a consensus sequence (Ala/Val-Met-Lys-Met) that is found in carboxylases
in most organisms. Numerous studies have indicated that HCS and BirA, as well as
biotin protein ligases from other organisms, can attach biotin to
apocarboxylases from different organisms, indicating that the mechanism of
biotin attachment is well conserved. In this study, we examined the
cross-reactivity of biotin attachment between human and bacterial biotin ligases
by comparing biotinylation of p-67 and BCCP87, the biotin-attachment domain
fragments from human propionyl-CoA carboxylase and E. coli acetyl-CoA
carboxylase, respectively. While BirA has similar biotinylation activity toward
the two substrates, HCS has reduced activity toward bacterial BCCP87 relative to
its native substrate, p-67. The crystal structure of a digested form of p-67,
spanning a sequence that contains a seven-residue protruding thumb loop in
BCCP87, revealed the absence of a similar structure in the human peptide.
Significantly, an engineered "thumbless" bacterial BCCP87 could be biotinylated
by HCS, with substrate affinity restored to near normal. This study suggests
that the thumb loop found in bacterial carboxylases interferes with optimal
interaction with the mammalian biotin protein ligase. While the function of the
thumb loop remains unknown, these results indicate a constraint on specificity
of the bacterial substrate for biotin attachment that is not itself a feature of
BirA.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

