 |
PDBsum entry 2hzp

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.7.1.3
- kynureninase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Tryptophan Catabolism
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-kynurenine + H2O = anthranilate + L-alanine + H+
|
 |
 |
 |
 |
 |
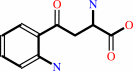 L-kynurenine
L-kynurenine
|
+
|
H2O
|
=
|
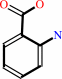 anthranilate
anthranilate
|
+
|
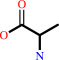 L-alanine
L-alanine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
46:2735-2744
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of Homo sapiens kynureninase.
|
|
S.Lima,
R.Khristoforov,
C.Momany,
R.S.Phillips.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Kynureninase is a member of a large family of catalytically diverse but
structurally homologous pyridoxal 5'-phosphate (PLP) dependent enzymes known as
the aspartate aminotransferase superfamily or alpha-family. The Homo sapiens and
other eukaryotic constitutive kynureninases preferentially catalyze the
hydrolytic cleavage of 3-hydroxy-l-kynurenine to produce 3-hydroxyanthranilate
and l-alanine, while l-kynurenine is the substrate of many prokaryotic inducible
kynureninases. The human enzyme was cloned with an N-terminal hexahistidine tag,
expressed, and purified from a bacterial expression system using Ni metal ion
affinity chromatography. Kinetic characterization of the recombinant enzyme
reveals classic Michaelis-Menten behavior, with a Km of 28.3 +/- 1.9 microM and
a specific activity of 1.75 micromol min-1 mg-1 for 3-hydroxy-dl-kynurenine.
Crystals of recombinant kynureninase that diffracted to 2.0 A were obtained, and
the atomic structure of the PLP-bound holoenzyme was determined by molecular
replacement using the Pseudomonas fluorescens kynureninase structure (PDB entry
1qz9) as the phasing model. A structural superposition with the P. fluorescens
kynureninase revealed that these two structures resemble the "open" and "closed"
conformations of aspartate aminotransferase. The comparison illustrates the
dynamic nature of these proteins' small domains and reveals a role for Arg-434
similar to its role in other AAT alpha-family members. Docking of
3-hydroxy-l-kynurenine into the human kynureninase active site suggests that
Asn-333 and His-102 are involved in substrate binding and molecular
discrimination between inducible and constitutive kynureninase substrates.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Y.Meng,
S.Katsuma,
K.Mita,
and
T.Shimada
(2009).
Abnormal red body coloration of the silkworm, Bombyx mori, is caused by a mutation in a novel kynureninase.
|
| |
Genes Cells,
14,
129-140.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

