 |
PDBsum entry 2dds

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Crystal structure of sphingomyelinase from bacillus cereus with cobalt ion
|
|
Structure:
|
 |
Sphingomyelin phosphodiesterase. Chain: a, b, c, d. Synonym: sphingomyelinase. Engineered: yes
|
|
Source:
|
 |
Bacillus cereus. Organism_taxid: 1396. Strain: iam 1029. Expressed in: bacillus subtilis. Expression_system_taxid: 1423.
|
|
Resolution:
|
 |
|
1.80Å
|
R-factor:
|
0.202
|
R-free:
|
0.232
|
|
|
Authors:
|
 |
H.Ago,M.Oda,M.Takahashi,H.Tsuge,S.Ochi,N.Katunuma,M.Miyano,J.Sakurai, Riken Structural Genomics/proteomics Initiative (Rsgi)
|
Key ref:

|
 |
H.Ago
et al.
(2006).
Structural basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus.
J Biol Chem,
281,
16157-16167.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
02-Feb-06
|
Release date:
|
02-May-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P11889
(PHL2_BACCE) -
Sphingomyelinase C from Bacillus cereus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
333 a.a.
299 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.4.12
- sphingomyelin phosphodiesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a sphingomyelin + H2O = phosphocholine + an N-acylsphing-4-enine + H+
|
 |
 |
 |
 |
 |
 sphingomyelin
sphingomyelin
|
+
|
H2O
|
=
|
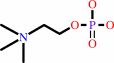 phosphocholine
phosphocholine
|
+
|
N-acylsphing-4-enine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
281:16157-16167
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus.
|
|
H.Ago,
M.Oda,
M.Takahashi,
H.Tsuge,
S.Ochi,
N.Katunuma,
M.Miyano,
J.Sakurai.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Sphingomyelinase (SMase) from Bacillus cereus (Bc-SMase) hydrolyzes
sphingomyelin to phosphocholine and ceramide in a divalent metal ion-dependent
manner. Bc-SMase is a homologue of mammalian neutral SMase (nSMase) and mimics
the actions of the endogenous mammalian nSMase in causing differentiation,
development, aging, and apoptosis. Thus Bc-SMase may be a good model for the
poorly characterized mammalian nSMase. The metal ion activation of
sphingomyelinase activity of Bc-SMase was in the order Co2+ > or = Mn2+ >
or = Mg2+ >> Ca2+ > or = Sr2+. The first crystal structures of Bc-SMase
bound to Co2+, Mg2+, or Ca2+ were determined. The water-bridged double divalent
metal ions at the center of the cleft in both the Co2+- and Mg2+-bound forms
were concluded to be the catalytic architecture required for sphingomyelinase
activity. In contrast, the architecture of Ca2+ binding at the site showed only
one binding site. A further single metal-binding site exists at one side edge of
the cleft. Based on the highly conserved nature of the residues of the binding
sites, the crystal structure of Bc-SMase with bound Mg2+ or Co2+ may provide a
common structural framework applicable to phosphohydrolases belonging to the
DNase I-like folding superfamily. In addition, the structural features and
site-directed mutagenesis suggest that the specific beta-hairpin with the
aromatic amino acid residues participates in binding to the membrane-bound
sphingomyelin substrate.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
The edge metal-binding site. The Mg^2+-bound form (A), the
Co^2+-bound form (B), and the Ca^2+-bound form (C) bind a
divalent metal ion each with octahedral geometry. The |F[o]| –
|F[c]| omit electron density map of the Mg^2+-bound form is
contoured at 4.5σ. The anomalous difference peaks of the Co^2+-
and the Ca^2+-bound forms were contoured at 5σ and 3σ,
respectively.
|
 |
Figure 3.
Effects of divalent metal ions on SM hydrolysis. The values
of V[max] and K[m] of hydrolytic activity for the dispersed SM
were measured using [N-methyl-^14C]SM. The measured result and
the ionic radius of each divalent cation are plotted against the
average Lewis acid strength (37). The plots with squares,
diamonds, and triangles are the plots of K[m], V[max], and the
ionic radius of each divalent cation.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2006,
281,
16157-16167)
copyright 2006.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.J.Clarke,
B.X.Wu,
and
Y.A.Hannun
(2011).
The neutral sphingomyelinase family: Identifying biochemical connections.
|
| |
Adv Enzyme Regul,
51,
51-58.
|
 |
|
|
|
|
 |
D.Sugimori,
Y.Tomita,
Y.Matsumoto,
and
C.Ogino
(2011).
Extracellular production of a sphingomyelinase from Streptomyces griseocarneus using Streptomyces lividans.
|
| |
Biotechnol Lett,
33,
727-731.
|
 |
|
|
|
|
 |
V.Lariccia,
M.Fine,
S.Magi,
M.J.Lin,
A.Yaradanakul,
M.C.Llaguno,
and
D.W.Hilgemann
(2011).
Massive calcium-activated endocytosis without involvement of classical endocytic proteins.
|
| |
J Gen Physiol,
137,
111-132.
|
 |
|
|
|
|
 |
D.Milhas,
C.J.Clarke,
and
Y.A.Hannun
(2010).
Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids.
|
| |
FEBS Lett,
584,
1887-1894.
|
 |
|
|
|
|
 |
M.J.Huseby,
A.C.Kruse,
J.Digre,
P.L.Kohler,
J.A.Vocke,
E.E.Mann,
K.W.Bayles,
G.A.Bohach,
P.M.Schlievert,
D.H.Ohlendorf,
and
C.A.Earhart
(2010).
Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms.
|
| |
Proc Natl Acad Sci U S A,
107,
14407-14412.
|
 |
|
|
|
|
 |
D.E.Saslowsky,
N.Tanaka,
K.P.Reddy,
and
W.I.Lencer
(2009).
Ceramide activates JNK to inhibit a cAMP-gated K+ conductance and Cl- secretion in intestinal epithelia.
|
| |
FASEB J,
23,
259-270.
|
 |
|
|
|
|
 |
M.Huseby,
K.Shi,
C.K.Brown,
J.Digre,
F.Mengistu,
K.S.Seo,
G.A.Bohach,
P.M.Schlievert,
D.H.Ohlendorf,
and
C.A.Earhart
(2007).
Structure and biological activities of beta toxin from Staphylococcus aureus.
|
| |
J Bacteriol,
189,
8719-8726.
|
 |
|
|
|
|
 |
R.L.Rich,
and
D.G.Myszka
(2007).
Survey of the year 2006 commercial optical biosensor literature.
|
| |
J Mol Recognit,
20,
300-366.
|
 |
|
|
|
|
 |
Y.Ramu,
Y.Xu,
and
Z.Lu
(2007).
Inhibition of CFTR Cl- channel function caused by enzymatic hydrolysis of sphingomyelin.
|
| |
Proc Natl Acad Sci U S A,
104,
6448-6453.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

