 |
PDBsum entry 2bwp

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
5-aminolevulinate synthase from rhodobacter capsulatus in complex with glycine
|
|
Structure:
|
 |
5-aminolevulinate synthase. Chain: a, b, d, e. Synonym: 5-aminolevulinic acid synthase, delta- aminolevulinate synthase, delta-ala synthetase. Engineered: yes
|
|
Source:
|
 |
Rhodobacter capsulatus. Organism_taxid: 1061. Atcc: 11166. Expressed in: escherichia coli. Expression_system_taxid: 562. Other_details: german collection of microorganisms (dsm) dsm 1710
|
|
Biol. unit:
|
 |
 Dimer (from PDB file)
Dimer (from PDB file)
|
|
Resolution:
|
 |
|
2.70Å
|
R-factor:
|
0.169
|
R-free:
|
0.220
|
|
|
Authors:
|
 |
I.Astner,J.O.Schulze,J.J.Van Den Heuvel,D.Jahn,W.-D.Schubert, D.W.Heinz
|
Key ref:

|
 |
I.Astner
et al.
(2005).
Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans.
EMBO J,
24,
3166-3177.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
15-Jul-05
|
Release date:
|
27-Sep-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P18079
(HEM1_RHOCB) -
5-aminolevulinate synthase from Rhodobacter capsulatus (strain ATCC BAA-309 / NBRC 16581 / SB1003)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
409 a.a.
398 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 5 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.3.1.37
- 5-aminolevulinate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Porphyrin Biosynthesis (early stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
succinyl-CoA + glycine + H+ = 5-aminolevulinate + CO2 + CoA
|
 |
 |
 |
 |
 |
succinyl-CoA
Bound ligand (Het Group name = )
matches with 80.00% similarity
|
+
|
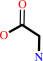 glycine
glycine
|
+
|
H(+)
|
=
|
 5-aminolevulinate
5-aminolevulinate
|
+
|
 CO2
CO2
|
+
|
 CoA
CoA
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLG)
matches with 71.43% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
EMBO J
24:3166-3177
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans.
|
|
I.Astner,
J.O.Schulze,
J.van den Heuvel,
D.Jahn,
W.D.Schubert,
D.W.Heinz.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
5-Aminolevulinate synthase (ALAS) is the first and rate-limiting enzyme of heme
biosynthesis in humans, animals, other non-plant eukaryotes, and
alpha-proteobacteria. It catalyzes the synthesis of 5-aminolevulinic acid, the
first common precursor of all tetrapyrroles, from glycine and succinyl-coenzyme
A (sCoA) in a pyridoxal 5'-phosphate (PLP)-dependent manner. X-linked
sideroblastic anemias (XLSAs), a group of severe disorders in humans
characterized by inadequate formation of heme in erythroblast mitochondria, are
caused by mutations in the gene for erythroid eALAS, one of two human genes for
ALAS. We present the first crystal structure of homodimeric ALAS from
Rhodobacter capsulatus (ALAS(Rc)) binding its cofactor PLP. We, furthermore,
present structures of ALAS(Rc) in complex with the substrates glycine or sCoA.
The sequence identity of ALAS from R. capsulatus and human eALAS is 49%.
XLSA-causing mutations may thus be mapped, revealing the molecular basis of XLSA
in humans. Mutations are found to obstruct substrate binding, disrupt the dimer
interface, or hamper the correct folding. The structure of ALAS completes the
structural analysis of enzymes in heme biosynthesis.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2 Schematic representation of the active site.
Glycine-bound PLP and sCoA are highlighted by bonds in black;
bonds of surrounding residues are shown in dark orange. Dotted
lines indicate hydrogen bonds (green numbers) and salt bridges
(red) between substrates or cofactors and ALAS; green
semicircles indicate hydrophobic interactions. An asterisk marks
residues from the second monomer. Lys248, involved in PLP
binding and catalysis, is marked by a blue box, residues
affected by mutations in human eALAS by a red box.
|
 |
Figure 6.
Figure 6 The reaction mechanism of ALAS. In the substrate-free
state, the cofactor PLP is bound by Lys248 (ALAS[Rc]), the
internal aldimine. Incoming glycine induces transaldimination,
leading to PLP binding glycine rather than Lys248 (external
aldimine). The substrate sCoA is nucleophilically attacked by
the PLP-activated glycine, leading to the addition of succinic
acid to glycine and concomitant loss of CoA. Decarboxylation
(carboxylate of the glycine moiety) of this intermediate yields
the PLP-bound product, released in the rate-limiting step of the
reaction.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
EMBO J
(2005,
24,
3166-3177)
copyright 2005.
|
|
| |
Figures were
selected
by the author.
|
|

|
| |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|

|
| |
Tetrapyrroles, such as hemes and chlorophylls are arguably among the most important pigments in the biosphere and the most versatile of cofactors.
The initial and rate-limiting enzyme of tetrapyrrole biosynthesis 5-aminolevulinate synthase (ALAS; E.C. 2.3.1.37) was discovered by Shemin and Neuberger in the late 1950's. ALAS catalyzes the PLP-dependent decarboxylative condensation of glycine and succinyl-coenzyme A. The product aminolevulinic acid (ALA) is the universal precursor of all tetrapyrroles.
Much later, ALAS was found to be restricted to non-plant eukaryotes and the gamma-group of proteobacteria. In plants and most bacteria, ALA is instead derived from tRNA-bound glutamate in two enzymatic steps via the C5-pathway involving glutamyl-tRNA reductase and glutamate-1-semialdehyde-2,1-aminomutase (GSAM). The originally discovered biosynthesis is known as the "Shemin pathway".
X-linked sideroblastic anemias (XLSA) are a group of severe disorders in humans, characterized by inadequate formation of heme in erythroblast mitochondria. They are caused by mutations in the gene for erythroid eALAS, one of two human genes for human ALAS.
The structure 2bwn is the first crystal structure ALAS. It is the homodimeric ALAS from the purple bacterium Rhodobacter capsulatus (ALAS-Rc) and includes the cofactor pyridoxal 5�-phosphate (PLP). 2bwo and 2bwp, respectively, are structures of ALAS-Rc in complex with the substrates glycine or succinyl-CoA. By combining the two, the active site immediately prior to the ensuing enzymatic reaction may be derived.
The sequence identity of ALAS from R. capsulatus and human eALAS is 49%. XLSA-causing mutations can therefore be mapped onto the bacterial protein to reveal the molecular basis of XLSA in humans. Mutations are mainly found to obstruct substrate binding, disrupt the dimer interface, or hamper the correct folding.
Wolf-Dieter Schubert
|
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Misumi,
T.Sugiura,
S.Yamaguchi,
T.Mori,
I.Kamei,
H.Hirai,
H.Kawagishi,
and
R.Kondo
(2011).
Cloning and transcriptional analysis of the gene encoding 5-aminolevulinic acid synthase of the white-rot fungus Phanerochaete sordida YK-624.
|
| |
Biosci Biotechnol Biochem,
75,
178-180.
|
 |
|
|
|
|
 |
A.K.Bergmann,
D.R.Campagna,
E.M.McLoughlin,
S.Agarwal,
M.D.Fleming,
S.S.Bottomley,
and
E.J.Neufeld
(2010).
Systematic molecular genetic analysis of congenital sideroblastic anemia: evidence for genetic heterogeneity and identification of novel mutations.
|
| |
Pediatr Blood Cancer,
54,
273-278.
|
 |
|
|
|
|
 |
G.Layer,
J.Reichelt,
D.Jahn,
and
D.W.Heinz
(2010).
Structure and function of enzymes in heme biosynthesis.
|
| |
Protein Sci,
19,
1137-1161.
|
 |
|
|
|
|
 |
M.C.Raman,
K.A.Johnson,
D.J.Clarke,
J.H.Naismith,
and
D.J.Campopiano
(2010).
The serine palmitoyltransferase from Sphingomonas wittichii RW1: An interesting link to an unusual acyl carrier protein.
|
| |
Biopolymers,
93,
811-822.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Zappa,
K.Li,
and
C.E.Bauer
(2010).
The tetrapyrrole biosynthetic pathway and its regulation in Rhodobacter capsulatus.
|
| |
Adv Exp Med Biol,
675,
229-250.
|
 |
|
|
|
|
 |
T.Lendrihas,
G.A.Hunter,
and
G.C.Ferreira
(2010).
Serine 254 enhances an induced fit mechanism in murine 5-aminolevulinate synthase.
|
| |
J Biol Chem,
285,
3351-3359.
|
 |
|
|
|
|
 |
W.Zhang,
M.L.Bolla,
D.Kahne,
and
C.T.Walsh
(2010).
A three enzyme pathway for 2-amino-3-hydroxycyclopent-2-enone formation and incorporation in natural product biosynthesis.
|
| |
J Am Chem Soc,
132,
6402-6411.
|
 |
|
|
|
|
 |
G.A.Hunter,
and
G.C.Ferreira
(2009).
5-aminolevulinate synthase: catalysis of the first step of heme biosynthesis.
|
| |
Cell Mol Biol (Noisy-le-grand),
55,
102-110.
|
 |
|
|
|
|
 |
M.C.Raman,
K.A.Johnson,
B.A.Yard,
J.Lowther,
L.G.Carter,
J.H.Naismith,
and
D.J.Campopiano
(2009).
The External Aldimine Form of Serine Palmitoyltransferase: STRUCTURAL, KINETIC, AND SPECTROSCOPIC ANALYSIS OF THE WILD-TYPE ENZYME AND HSAN1 MUTANT MIMICS.
|
| |
J Biol Chem,
284,
17328-17339.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Macieira,
J.Zhang,
M.Velarde,
W.Buckel,
and
A.Messerschmidt
(2009).
Crystal structure of 4-hydroxybutyrate CoA-transferase from Clostridium aminobutyricum.
|
| |
Biol Chem,
390,
1251-1263.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Lendrihas,
J.Zhang,
G.A.Hunter,
and
G.C.Ferreira
(2009).
Arg-85 and Thr-430 in murine 5-aminolevulinate synthase coordinate acyl-CoA-binding and contribute to substrate specificity.
|
| |
Protein Sci,
18,
1847-1859.
|
 |
|
|
|
|
 |
Y.Shiraiwa,
H.Ikushiro,
and
H.Hayashi
(2009).
Multifunctional role of his159in the catalytic reaction of serine palmitoyltransferase.
|
| |
J Biol Chem,
284,
15487-15495.
|
 |
|
|
|
|
 |
C.Camaschella
(2008).
Recent advances in the understanding of inherited sideroblastic anaemia.
|
| |
Br J Haematol,
143,
27-38.
|
 |
|
|
|
|
 |
H.Ikushiro,
S.Fujii,
Y.Shiraiwa,
and
H.Hayashi
(2008).
Acceleration of the substrate Calpha deprotonation by an analogue of the second substrate palmitoyl-CoA in Serine Palmitoyltransferase.
|
| |
J Biol Chem,
283,
7542-7553.
|
 |
|
|
|
|
 |
P.F.Larsen,
E.E.Nielsen,
T.D.Williams,
and
V.Loeschcke
(2008).
Intraspecific variation in expression of candidate genes for osmoregulation, heme biosynthesis and stress resistance suggests local adaptation in European flounder (Platichthys flesus).
|
| |
Heredity,
101,
247-259.
|
 |
|
|
|
|
 |
S.D.Whatley,
S.Ducamp,
L.Gouya,
B.Grandchamp,
C.Beaumont,
M.N.Badminton,
G.H.Elder,
S.A.Holme,
A.V.Anstey,
M.Parker,
A.V.Corrigall,
P.N.Meissner,
R.J.Hift,
J.T.Marsden,
Y.Ma,
G.Mieli-Vergani,
J.C.Deybach,
and
H.Puy
(2008).
C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload.
|
| |
Am J Hum Genet,
83,
408-414.
|
 |
|
|
|
|
 |
G.A.Hunter,
J.Zhang,
and
G.C.Ferreira
(2007).
Transient kinetic studies support refinements to the chemical and kinetic mechanisms of aminolevulinate synthase.
|
| |
J Biol Chem,
282,
23025-23035.
|
 |
|
|
|
|
 |
H.Ikushiro,
M.M.Islam,
H.Tojo,
and
H.Hayashi
(2007).
Molecular characterization of membrane-associated soluble serine palmitoyltransferases from Sphingobacterium multivorum and Bdellovibrio stolpii.
|
| |
J Bacteriol,
189,
5749-5761.
|
 |
|
|
|
|
 |
T.D.Turbeville,
J.Zhang,
G.A.Hunter,
and
G.C.Ferreira
(2007).
Histidine 282 in 5-aminolevulinate synthase affects substrate binding and catalysis.
|
| |
Biochemistry,
46,
5972-5981.
|
 |
|
|
|
|
 |
A.S.Tsiftsoglou,
A.I.Tsamadou,
and
L.C.Papadopoulou
(2006).
Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects.
|
| |
Pharmacol Ther,
111,
327-345.
|
 |
|
|
|
|
 |
D.Alexeev,
R.L.Baxter,
D.J.Campopiano,
O.Kerbarh,
L.Sawyer,
N.Tomczyk,
R.Watt,
and
S.P.Webster
(2006).
Suicide inhibition of alpha-oxamine synthases: structures of the covalent adducts of 8-amino-7-oxononanoate synthase with trifluoroalanine.
|
| |
Org Biomol Chem,
4,
1209-1212.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Fontenay,
S.Cathelin,
M.Amiot,
E.Gyan,
and
E.Solary
(2006).
Mitochondria in hematopoiesis and hematological diseases.
|
| |
Oncogene,
25,
4757-4767.
|
 |
|
|
|
|
 |
M.Petrícek,
K.Petrícková,
L.Havlícek,
and
J.Felsberg
(2006).
Occurrence of two 5-aminolevulinate biosynthetic pathways in Streptomyces nodosus subsp. asukaensis is linked with the production of asukamycin.
|
| |
J Bacteriol,
188,
5113-5123.
|
 |
|
|
|
|
 |
V.M.Bhor,
S.Dev,
G.R.Vasanthakumar,
P.Kumar,
S.Sinha,
and
A.Surolia
(2006).
Broad substrate stereospecificity of the Mycobacterium tuberculosis 7-keto-8-aminopelargonic acid synthase: Spectroscopic and kinetic studies.
|
| |
J Biol Chem,
281,
25076-25088.
|
 |
|
|
|
|
 |
X.Lv,
J.Fan,
H.Ge,
Y.Gao,
X.Zhang,
M.Teng,
and
L.Niu
(2006).
Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of the glutamate-1-semialdehyde aminotransferase from Bacillus subtilis.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
483-485.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

