 |
PDBsum entry 2gdv

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.4.1.7
- sucrose phosphorylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
sucrose + phosphate = D-fructose + alpha-D-glucose 1-phosphate
|
 |
 |
 |
 |
 |
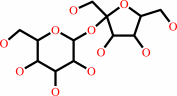 sucrose
sucrose
|
+
|
 phosphate
phosphate
|
=
|
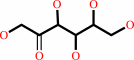 D-fructose
D-fructose
|
+
|
alpha-D-glucose 1-phosphate
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
281:35576-35584
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural rearrangements of sucrose phosphorylase from Bifidobacterium adolescentis during sucrose conversion.
|
|
O.Mirza,
L.K.Skov,
D.Sprogøe,
L.A.van den Broek,
G.Beldman,
J.S.Kastrup,
M.Gajhede.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The reaction mechanism of sucrose phosphorylase from Bifidobacterium
adolescentis (BiSP) was studied by site-directed mutagenesis and x-ray
crystallography. An inactive mutant of BiSP (E232Q) was co-crystallized with
sucrose. The structure revealed a substrate-binding mode comparable with that
seen in other related sucrose-acting enzymes. Wild-type BiSP was also
crystallized in the presence of sucrose. In the dimeric structure, a covalent
glucosyl intermediate was formed in one molecule of the BiSP dimer, and after
hydrolysis of the glucosyl intermediate, a beta-D-glucose product complex was
formed in the other molecule. Although the overall structure of the
BiSP-glucosyl intermediate complex is similar to that of the BiSP(E232Q)-sucrose
complex, the glucose complex discloses major differences in loop conformations.
Two loops (residues 336-344 and 132-137) in the proximity of the active site
move up to 16 and 4 A, respectively. On the basis of these findings, we have
suggested a reaction cycle that takes into account the large movements in the
active-site entrance loops.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIGURE 1. Schematic representation of the domain
organization of the mixed dimer of BiSP. The glucosyl
intermediate (molecule A; left) and the glucose hydrolysis
product (molecule B; right) are shown as red spheres. domains A,
B, B', and C are colored green, yellow, blue, and orange,
respectively. N and C correspond to the N and C termini,
respectively, whereas A and B indicate the positions of loops A
and B.
|
 |
Figure 4.
FIGURE 4. Structural changes occurring during the enzyme
reaction. A, close-up view of loops A and B of the wild-type
BiSP covalent intermediate (molecule A; cyan) superimposed on
the glucose product-bound form (molecule B; yellow). The bound
glucose of molecule B is shown for clarity. B, close-up view of
loops A and B and the noncovalently bound glucose molecule of
the wild-type BiSP-glucose complex. Glucose 1-phosphate (yellow)
has been modeled based on the position of the glucose
interacting with Arg^135 and Tyr^344. C, proposed intermolecular
phosphate-binding site created by two Arg^135 residues. The
distances indicated by dashed lines are 3.9 Å. The bound
sucrose molecules are shown as red van der Waals spheres.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2006,
281,
35576-35584)
copyright 2006.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Goedl,
and
B.Nidetzky
(2009).
Sucrose phosphorylase harbouring a redesigned, glycosyltransferase-like active site exhibits retaining glucosyl transfer in the absence of a covalent intermediate.
|
| |
Chembiochem,
10,
2333-2337.
|
 |
|
|
|
|
 |
K.Nomura,
K.Sugimoto,
H.Nishiura,
K.Ohdan,
T.Nishimura,
H.Hayashi,
and
T.Kuriki
(2008).
Glucosylation of acetic acid by sucrose phosphorylase.
|
| |
Biosci Biotechnol Biochem,
72,
82-87.
|
 |
|
|
|
|
 |
L.A.van den Broek,
S.W.Hinz,
G.Beldman,
J.P.Vincken,
and
A.G.Voragen
(2008).
Bifidobacterium carbohydrases-their role in breakdown and synthesis of (potential) prebiotics.
|
| |
Mol Nutr Food Res,
52,
146-163.
|
 |
|
|
|
|
 |
S.Guglielmetti,
I.Tamagnini,
D.Mora,
M.Minuzzo,
A.Scarafoni,
S.Arioli,
J.Hellman,
M.Karp,
and
C.Parini
(2008).
Implication of an outer surface lipoprotein in adhesion of Bifidobacterium bifidum to Caco-2 cells.
|
| |
Appl Environ Microbiol,
74,
4695-4702.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

