 |
PDBsum entry 1w2g

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.4.9
- dTMP kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
dTMP + ATP = dTDP + ADP
|
 |
 |
 |
 |
 |
 dTMP
dTMP
|
+
|
ATP
Bound ligand (Het Group name = )
matches with 80.95% similarity
|
=
|
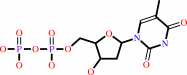 dTDP
dTDP
|
+
|
 ADP
ADP
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
44:130-137
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
The crystal structure of Mycobacterium tuberculosis thymidylate kinase in complex with 3'-azidodeoxythymidine monophosphate suggests a mechanism for competitive inhibition.
|
|
E.Fioravanti,
V.Adam,
H.Munier-Lehmann,
D.Bourgeois.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Tuberculosis (TB) is the primary cause of mortality among infectious diseases.
Mycobacterium tuberculosis thymidylate kinase (TMPK(Mtub)) catalyzes the
ATP-dependent phosphorylation of deoxythymidine 5'-monophosphate (dTMP).
Essential to DNA replication, this enzyme represents a promising target for
developing new drugs against TB, because the configuration of its active site is
unique within the TMPK family. Indeed, it has been proposed that, as opposed to
other TMPKs, catalysis by TMPK(Mtub) necessitates the transient binding of a
magnesium ion coordinating the phosphate acceptor. Moreover,
3'-azidodeoxythymidine monophosphate (AZTMP) is a competitive inhibitor of
TMPK(Mtub), whereas it is a substrate for human and other TMPKs. Here, the
crystal structures of TMPK(Mtub) in complex with deoxythymidine (dT) and AZTMP
were determined to 2.1 and 2.0 A resolution, respectively, and suggest a
mechanism for inhibition. The azido group of AZTMP perturbs the induced-fit
mechanism normally adopted by the enzyme. Magnesium is prevented from binding,
and the resulting electrostatic environment precludes phosphoryl transfer from
occurring. Our data provide a model for drug development against tuberculosis.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Carnrot,
L.Wang,
D.Topalis,
and
S.Eriksson
(2008).
Mechanisms of substrate selectivity for Bacillus anthracis thymidylate kinase.
|
| |
Protein Sci,
17,
1486-1493.
|
 |
|
|
|
|
 |
O.Familiar,
H.Munier-Lehmann,
A.Negri,
F.Gago,
D.Douguet,
L.Rigouts,
A.I.Hernández,
M.J.Camarasa,
and
M.J.Pérez-Pérez
(2008).
Exploring acyclic nucleoside analogues as inhibitors of Mycobacterium tuberculosis thymidylate kinase.
|
| |
ChemMedChem,
3,
1083-1093.
|
 |
|
|
|
|
 |
N.Couto,
M.F.Duarte,
M.T.Fernandez,
P.Rodrigues,
M.T.Barros,
M.L.Costa,
and
B.J.Cabral
(2007).
Complexation of transition metals by 3-azidopropionitrile. An electrospray ionization mass spectrometry study.
|
| |
J Am Soc Mass Spectrom,
18,
453-465.
|
 |
|
|
|
|
 |
G.Hible,
P.Christova,
L.Renault,
E.Seclaman,
A.Thompson,
E.Girard,
H.Munier-Lehmann,
and
J.Cherfils
(2006).
Unique GMP-binding site in Mycobacterium tuberculosis guanosine monophosphate kinase.
|
| |
Proteins,
62,
489-500.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

