 |
PDBsum entry 1vap

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Lipid degradation
|
PDB id
|
|

|

|
1vap
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
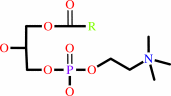 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
272:3573-3582
(1997)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural aspects of interfacial adsorption. A crystallographic and site-directed mutagenesis study of the phospholipase A2 from the venom of Agkistrodon piscivorus piscivorus.
|
|
S.K.Han,
E.T.Yoon,
D.L.Scott,
P.B.Sigler,
W.Cho.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Recent genetic and structural studies have shed considerable light on the
mechanism by which secretory phospholipases A2 interact with substrate
aggregates. Electrostatic forces play an essential role in optimizing
interfacial catalysis. Efficient and productive adsorption of the Class I bovine
pancreatic phospholipase A2 to anionic interfaces is dependent upon the presence
of two nonconserved lysine residues at sequence positions 56 and 116, implying
that critical components of the adsorption surface differ among enzyme species
(Dua, R., Wu, S.-K., and Cho, W. (1995) J. Biol. Chem. 270, 263-268). In an
effort to further characterize the protein residues involved in interfacial
catalysis, we have determined the high resolution (1.7 A) x-ray structure of the
Class II Asp-49 phospholipase A2 from the venom of Agkistrodon piscivorus
piscivorus. Correlation of the three-dimensional coordinates with kinetic data
derived from site-directed mutations near the amino terminus (E6R, K7E, K10E,
K11E, and K16E) and the active site (K54E and K69Y) defines much of the
interface topography. Lysine residues at sequence positions 7 and 10 mediate the
adsorption of A. p. piscivorus phospholipase A2 to anionic interfaces but play
little role in the enzyme's interaction with electrically neutral surfaces or in
substrate binding. Compared to the native enzyme, the mutant proteins K7E and
K10E demonstrate comparable (20-fold) decreases in affinity and catalysis on
polymerized mixed liposomes of
1-hexadecanoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphocholine and
1,2-bis[12-(lipoyloxy)dodecanoyl]-sn-glycero-3-phosphoglycerol, while the double
mutant, K7E/K10E, shows a more dramatic 500-fold decrease in catalysis and
interfacial adsorption. The calculated contributions of Lys-7 and Lys-10 to the
free energy of binding of A. p. piscivorus phospholipase A2 to anionic liposomes
(-1.8 kcal/mol at 25 degrees C per lysine) are additive (i.e. -3.7 kcal/mol) and
together represent nearly half of the total binding energy. Although both lysine
side chains lie exposed at the edge of the proposed interfacial adsorption
surface, they are geographically remote from the corresponding interfacial
determinants for the bovine enzyme. Our results confirm that interfacial
adsorption is largely driven by electrostatic forces and demonstrate that the
arrangement of the critical charges (e.g. lysines) is species-specific. This
variability in the topography of the adsorption surface suggests a corresponding
flexibility in the orientation of the active enzyme at the substrate interface.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig. 2. A stereoview of the  -carbon
trace of the crystalline App-D49 indicating the positions of
mutated residues. The view of the enzyme shown here is similar
to that used in previous publications to illustrate the location
of a co-crystallized transition-state^ analog (9, 17, 28). The
active site lies at the base of^ the central cavity formed from
the amino-terminal helix, residues 19-23, portions of the
calcium-binding loop, and the side chain of Lys-69 and is
indicated by the side chain of His-48 (in black). The plane of
the putative interfacial adsorption surface lies perpendicular
to the hydrophobic channel and incorporates residues surrounding
the external opening of the channel. In the present study,
specific lysine residues (Lys-7, Lys-10, Lys-11, Lys-16, Lys-54,
and Lys-69) were changed into glutamates and tyrosine^ (Lys-69)
in an effort to characterize the structural determinants of
interfacial adsorption. -carbon
trace of the crystalline App-D49 indicating the positions of
mutated residues. The view of the enzyme shown here is similar
to that used in previous publications to illustrate the location
of a co-crystallized transition-state^ analog (9, 17, 28). The
active site lies at the base of^ the central cavity formed from
the amino-terminal helix, residues 19-23, portions of the
calcium-binding loop, and the side chain of Lys-69 and is
indicated by the side chain of His-48 (in black). The plane of
the putative interfacial adsorption surface lies perpendicular
to the hydrophobic channel and incorporates residues surrounding
the external opening of the channel. In the present study,
specific lysine residues (Lys-7, Lys-10, Lys-11, Lys-16, Lys-54,
and Lys-69) were changed into glutamates and tyrosine^ (Lys-69)
in an effort to characterize the structural determinants of
interfacial adsorption.
|
 |
Figure 5.
Fig. 5. The interaction of a transition-state analog
(L-1-O-octyl-2-heptylphosphonyl-sn-glycero-3-phosphoethanolamine)
with the^ active site of the Class I PLA[2] from the venom of N.
n. atra (A). Class II PLA[2]s, including App-D49, substitute a
lysine residue^ for the tyrosine at sequence position 69. The
K69Y mutant has essentially the same activity as the wild type
enzyme toward PC^ and PE substrates but shows a 3-fold drop in
activity toward PG substrate. One explanation for this finding
is that the  -ammonium
group of Lys-69 forms additional hydrogen bonds with
phospholipid^ head groups, especially with PG whose hydroxyl
groups can function as hydrogen bond acceptors (B). Such an
interaction would not be achievable by the phenolic oxygen of
Tyr-69 or with PC and^ PE as substrate. -ammonium
group of Lys-69 forms additional hydrogen bonds with
phospholipid^ head groups, especially with PG whose hydroxyl
groups can function as hydrogen bond acceptors (B). Such an
interaction would not be achievable by the phenolic oxygen of
Tyr-69 or with PC and^ PE as substrate.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(1997,
272,
3573-3582)
copyright 1997.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Kadoshima-Yamaoka,
M.Goto,
M.Murakawa,
R.Yoshioka,
Y.Tanaka,
H.Inoue,
H.Murafuji,
S.Kanki,
Y.Hayashi,
K.Nagahira,
A.Ogata,
T.Nakatsuka,
and
Y.Fukuda
(2009).
ASB16165, a phosphodiesterase 7A inhibitor, reduces cutaneous TNF-alpha level and ameliorates skin edema in phorbol ester 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation model in mice.
|
| |
Eur J Pharmacol,
613,
163-166.
|
 |
|
|
|
|
 |
G.Lambeau,
and
M.H.Gelb
(2008).
Biochemistry and physiology of mammalian secreted phospholipases A2.
|
| |
Annu Rev Biochem,
77,
495-520.
|
 |
|
|
|
|
 |
L.Linderoth,
T.L.Andresen,
K.Jørgensen,
R.Madsen,
and
G.H.Peters
(2008).
Molecular basis of phospholipase A2 activity toward phospholipids with sn-1 substitutions.
|
| |
Biophys J,
94,
14-26.
|
 |
|
|
|
|
 |
R.Malik,
R.S.Bora,
D.Gupta,
P.Sharma,
R.Arya,
S.Chaudhary,
and
K.S.Saini
(2008).
Cloning, stable expression of human phosphodiesterase 7A and development of an assay for screening of PDE7 selective inhibitors.
|
| |
Appl Microbiol Biotechnol,
77,
1167-1173.
|
 |
|
|
|
|
 |
G.Faure,
V.T.Gowda,
and
R.C.Maroun
(2007).
Characterization of human coagulation factor Xa-binding site on Viperidae snake venom phospholipases A2 by affinity binding studies and molecular bioinformatics.
|
| |
BMC Struct Biol,
7,
82.
|
 |
|
|
|
|
 |
J.N.Fleming-Waddell,
L.M.Wilson,
G.R.Olbricht,
T.Vuocolo,
K.Byrne,
B.A.Craig,
R.L.Tellam,
N.E.Cockett,
and
C.A.Bidwell
(2007).
Analysis of gene expression during the onset of muscle hypertrophy in callipyge lambs.
|
| |
Anim Genet,
38,
28-36.
|
 |
|
|
|
|
 |
L.Vijayakrishnan,
S.Rudra,
M.S.Eapen,
S.Dastidar,
and
A.Ray
(2007).
Small-molecule inhibitors of PDE-IV and -VII in the treatment of respiratory diseases and chronic inflammation.
|
| |
Expert Opin Investig Drugs,
16,
1585-1599.
|
 |
|
|
|
|
 |
C.Leidy,
L.Linderoth,
T.L.Andresen,
O.G.Mouritsen,
K.Jørgensen,
and
G.H.Peters
(2006).
Domain-induced activation of human phospholipase A2 type IIA: local versus global lipid composition.
|
| |
Biophys J,
90,
3165-3175.
|
 |
|
|
|
|
 |
C.Lugnier
(2006).
Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents.
|
| |
Pharmacol Ther,
109,
366-398.
|
 |
|
|
|
|
 |
S.Uckert,
P.Hedlund,
K.E.Andersson,
M.C.Truss,
U.Jonas,
and
C.G.Stief
(2006).
Update on phosphodiesterase (PDE) isoenzymes as pharmacologic targets in urology: present and future.
|
| |
Eur Urol,
50,
1194.
|
 |
|
|
|
|
 |
S.Uckert,
C.G.Stief,
M.Mayer,
U.Jonas,
and
P.Hedlund
(2005).
Distribution and functional significance of phosphodiesterase isoenzymes in the human lower urinary tract.
|
| |
World J Urol,
23,
368-373.
|
 |
|
|
|
|
 |
T.L.Andresen,
S.S.Jensen,
and
K.Jørgensen
(2005).
Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release.
|
| |
Prog Lipid Res,
44,
68-97.
|
 |
|
|
|
|
 |
M.Perbandt,
I.H.Tsai,
A.Fuchs,
S.Banumathi,
K.R.Rajashankar,
D.Georgieva,
N.Kalkura,
T.P.Singh,
N.Genov,
and
C.Betzel
(2003).
Structure of the heterodimeric neurotoxic complex viperotoxin F (RV-4/RV-7) from the venom of Vipera russelli formosensis at 1.9 A resolution.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
1679-1687.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.A.Tatulian
(2003).
Structural effects of covalent inhibition of phospholipase A2 suggest allosteric coupling between membrane binding and catalytic sites.
|
| |
Biophys J,
84,
1773-1783.
|
 |
|
|
|
|
 |
A.H.de Oliveira,
J.R.Giglio,
S.H.Andrião-Escarso,
A.S.Ito,
and
R.J.Ward
(2001).
A pH-induced dissociation of the dimeric form of a lysine 49-phospholipase A2 abolishes Ca2+-independent membrane damaging activity.
|
| |
Biochemistry,
40,
6912-6920.
|
 |
|
|
|
|
 |
B.Lathrop,
M.Gadd,
R.L.Biltonen,
and
G.S.Rule
(2001).
Changes in Ca2+ affinity upon activation of Agkistrodon piscivorus piscivorus phospholipase A2.
|
| |
Biochemistry,
40,
3264-3272.
|
 |
|
|
|
|
 |
K.Yuasa,
T.Ohgaru,
M.Asahina,
and
K.Omori
(2001).
Identification of rat cyclic nucleotide phosphodiesterase 11A (PDE11A): comparison of rat and human PDE11A splicing variants.
|
| |
Eur J Biochem,
268,
4440-4448.
|
 |
|
|
|
|
 |
M.T.Hyvönen,
K.Oörni,
P.T.Kovanen,
and
M.Ala-Korpela
(2001).
Changes in a phospholipid bilayer induced by the hydrolysis of a phospholipase A2 enzyme: a molecular dynamics simulation study.
|
| |
Biophys J,
80,
565-578.
|
 |
|
|
|
|
 |
R.V.Stahelin,
and
W.Cho
(2001).
Differential roles of ionic, aliphatic, and aromatic residues in membrane-protein interactions: a surface plasmon resonance study on phospholipases A2.
|
| |
Biochemistry,
40,
4672-4678.
|
 |
|
|
|
|
 |
S.A.Tatulian
(2001).
Toward understanding interfacial activation of secretory phospholipase A2 (PLA2): membrane surface properties and membrane-induced structural changes in the enzyme contribute synergistically to PLA2 activation.
|
| |
Biophys J,
80,
789-800.
|
 |
|
|
|
|
 |
W.H.Lee,
M.T.da Silva Giotto,
S.Marangoni,
M.H.Toyama,
I.Polikarpov,
and
R.C.Garratt
(2001).
Structural basis for low catalytic activity in Lys49 phospholipases A2--a hypothesis: the crystal structure of piratoxin II complexed to fatty acid.
|
| |
Biochemistry,
40,
28-36.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Dessen
(2000).
Phospholipase A(2) enzymes: structural diversity in lipid messenger metabolism.
|
| |
Structure,
8,
R15-R22.
|
 |
|
|
|
|
 |
M.Falconi,
A.Desideri,
and
S.Rufini
(2000).
Membrane-perturbing activity of Viperidae myotoxins: an electrostatic surface potential approach to a puzzling problem.
|
| |
J Mol Recognit,
13,
14-19.
|
 |
|
|
|
|
 |
N.Iijima,
S.Uchiyama,
Y.Fujikawa,
and
M.Esaka
(2000).
Purification, characterization, and molecular cloning of group I phospholipases A2 from the gills of the red sea bream, Pagrus major.
|
| |
Lipids,
35,
1359-1370.
|
 |
|
|
|
|
 |
B.I.Lee,
R.Dua,
and
W.Cho
(1999).
A structural determinant of the unique interfacial binding mode of bovine pancreatic phospholipase A2.
|
| |
Biochemistry,
38,
7811-7818.
|
 |
|
|
|
|
 |
F.Reichsman,
H.M.Moore,
and
S.Cumberledge
(1999).
Sequence homology between Wingless/Wnt-1 and a lipid-binding domain in secreted phospholipase A2.
|
| |
Curr Biol,
9,
R353-R355.
|
 |
|
|
|
|
 |
J.Kotera,
K.Fujishige,
Y.Imai,
E.Kawai,
H.Michibata,
H.Akatsuka,
N.Yanaka,
and
K.Omori
(1999).
Genomic origin and transcriptional regulation of two variants of cGMP-binding cGMP-specific phosphodiesterases.
|
| |
Eur J Biochem,
262,
866-873.
|
 |
|
|
|
|
 |
K.Fujishige,
J.Kotera,
and
K.Omori
(1999).
Striatum- and testis-specific phosphodiesterase PDE10A isolation and characterization of a rat PDE10A.
|
| |
Eur J Biochem,
266,
1118-1127.
|
 |
|
|
|
|
 |
M.Ahlström,
and
C.Lamberg-Allardt
(1999).
Regulation of adenosine 3',5'-cyclic monophosphate (cAMP) accumulation in UMR-106 osteoblast-like cells: role of cAMP-phosphodiesterase and cAMP efflux.
|
| |
Biochem Pharmacol,
58,
1335-1340.
|
 |
|
|
|
|
 |
M.H.Gelb,
W.Cho,
and
D.C.Wilton
(1999).
Interfacial binding of secreted phospholipases A(2): more than electrostatics and a major role for tryptophan.
|
| |
Curr Opin Struct Biol,
9,
428-432.
|
 |
|
|
|
|
 |
M.J.Janssen,
L.Vermeulen,
H.A.Van der Helm,
A.J.Aarsman,
A.J.Slotboom,
and
M.R.Egmond
(1999).
Enzymatic properties of rat group IIA and V phospholipases A(2) compared.
|
| |
Biochim Biophys Acta,
1440,
59-72.
|
 |
|
|
|
|
 |
M.Sumandea,
S.Das,
C.Sumandea,
and
W.Cho
(1999).
Roles of aromatic residues in high interfacial activity of Naja naja atra phospholipase A2.
|
| |
Biochemistry,
38,
16290-16297.
|
 |
|
|
|
|
 |
T.P.Dousa
(1999).
Cyclic-3',5'-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney.
|
| |
Kidney Int,
55,
29-62.
|
 |
|
|
|
|
 |
Y.Snitko,
S.K.Han,
B.I.Lee,
and
W.Cho
(1999).
Differential interfacial and substrate binding modes of mammalian pancreatic phospholipases A2: a comparison among human, bovine, and porcine enzymes.
|
| |
Biochemistry,
38,
7803-7810.
|
 |
|
|
|
|
 |
F.Ghomashchi,
Y.Lin,
M.S.Hixon,
B.Z.Yu,
R.Annand,
M.K.Jain,
and
M.H.Gelb
(1998).
Interfacial recognition by bee venom phospholipase A2: insights into nonelectrostatic molecular determinants by charge reversal mutagenesis.
|
| |
Biochemistry,
37,
6697-6710.
|
 |
|
|
|
|
 |
J.B.Henshaw,
C.A.Olsen,
A.R.Farnbach,
K.H.Nielson,
and
J.D.Bell
(1998).
Definition of the specific roles of lysolecithin and palmitic acid in altering the susceptibility of dipalmitoylphosphatidylcholine bilayers to phospholipase A2.
|
| |
Biochemistry,
37,
10709-10721.
|
 |
|
|
|
|
 |
L.Lichtenbergova,
E.T.Yoon,
and
W.Cho
(1998).
Membrane penetration of cytosolic phospholipase A2 is necessary for its interfacial catalysis and arachidonate specificity.
|
| |
Biochemistry,
37,
14128-14136.
|
 |
|
|
|
|
 |
M.J.Perry,
and
G.A.Higgs
(1998).
Chemotherapeutic potential of phosphodiesterase inhibitors.
|
| |
Curr Opin Chem Biol,
2,
472-481.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

