 |
PDBsum entry 1szu

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
The structure of gamma-aminobutyrate aminotransferase mutant: v241a
|
|
Structure:
|
 |
4-aminobutyrate aminotransferase. Chain: a, b, c, d. Synonym: gamma-amino-n-butyrate transaminase, gaba transaminase, glutamate:succinic semialdehyde transaminase, gaba aminotransferase, gaba-at. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Gene: gabt, b2662. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Biol. unit:
|
 |
Tetramer (from
)
|
|
Resolution:
|
 |
|
2.52Å
|
R-factor:
|
0.154
|
R-free:
|
0.202
|
|
|
Authors:
|
 |
W.Liu,P.E.Peterson,J.A.Langston,X.Jin,X.Zhou,A.J.Fisher,M.D.Toney
|
Key ref:

|
 |
W.Liu
et al.
(2005).
Kinetic and crystallographic analysis of active site mutants of Escherichia coli gamma-aminobutyrate aminotransferase.
Biochemistry,
44,
2982-2992.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
06-Apr-04
|
Release date:
|
01-Mar-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P22256
(GABT_ECOLI) -
4-aminobutyrate aminotransferase GabT from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
426 a.a.
425 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.6.1.19
- 4-aminobutyrate--2-oxoglutarate transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
4-aminobutanoate + 2-oxoglutarate = succinate semialdehyde + L-glutamate
|
 |
 |
 |
 |
 |
 4-aminobutanoate
4-aminobutanoate
|
+
|
2-oxoglutarate
Bound ligand (Het Group name = )
matches with 40.00% similarity
|
=
|
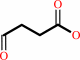 succinate semialdehyde
succinate semialdehyde
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
Enzyme class 2:
|
 |
E.C.2.6.1.48
- 5-aminovalerate transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
5-aminopentanoate + 2-oxoglutarate = 5-oxopentanoate + L-glutamate
|
 |
 |
 |
 |
 |
 5-aminopentanoate
5-aminopentanoate
|
+
|
2-oxoglutarate
Bound ligand (Het Group name = )
matches with 40.00% similarity
|
=
|
 5-oxopentanoate
5-oxopentanoate
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
44:2982-2992
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Kinetic and crystallographic analysis of active site mutants of Escherichia coli gamma-aminobutyrate aminotransferase.
|
|
W.Liu,
P.E.Peterson,
J.A.Langston,
X.Jin,
X.Zhou,
A.J.Fisher,
M.D.Toney.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The E. coli isozyme of gamma-aminobutyrate aminotransferase (GABA-AT) is a
tetrameric pyridoxal phosphate-dependent enzyme that catalyzes transamination
between primary amines and alpha-keto acids. The roles of the active site
residues V241, E211, and I50 in the GABA-AT mechanism have been probed by
site-directed mutagenesis. The beta-branched side chain of V241 facilitates
formation of external aldimine intermediates with primary amine substrates,
while E211 provides charge compensation of R398 selectively in the primary amine
half-reaction and I50 forms a hydrophobic lid at the top of the substrate
binding site. The structures of the I50Q, V241A, and E211S mutants were solved
by X-ray crystallography to resolutions of 2.1, 2.5, and 2.52 A, respectively.
The structure of GABA-AT is similar in overall fold and active site structure to
that of dialkylglycine decarboxylase, which catalyzes both transamination and
decarboxylation half-reactions in its normal catalytic cycle. Therefore, an
attempt was made to convert GABA-AT into a decarboxylation-dependent
aminotransferase similar to dialkylglycine decarboxylase by systematic mutation
of E. coli GABA-AT active site residues. Two of the twelve mutants presented,
E211S/I50G/C77K and E211S/I50H/V80D, have approximately 10-fold higher
decarboxylation activities than the wild-type enzyme, and the E211S/I50H/V80D
has formally changed the reaction specificity to that of a decarboxylase.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.Kurihara,
K.Kato,
K.Asada,
H.Kumagai,
and
H.Suzuki
(2010).
A putrescine-inducible pathway comprising PuuE-YneI in which gamma-aminobutyrate is degraded into succinate in Escherichia coli K-12.
|
| |
J Bacteriol,
192,
4582-4591.
|
 |
|
|
|
|
 |
C.Gross,
R.Felsheim,
and
L.P.Wackett
(2008).
Genes and enzymes of azetidine-2-carboxylate metabolism: detoxification and assimilation of an antibiotic.
|
| |
J Bacteriol,
190,
4859-4864.
|
 |
|
|
|
|
 |
G.Andersen,
B.Andersen,
D.Dobritzsch,
K.D.Schnackerz,
and
J.Piskur
(2007).
A gene duplication led to specialized gamma-aminobutyrate and beta-alanine aminotransferase in yeast.
|
| |
FEBS J,
274,
1804-1817.
|
 |
|
|
|
|
 |
B.Julien,
Z.Q.Tian,
R.Reid,
and
C.D.Reeves
(2006).
Analysis of the ambruticin and jerangolid gene clusters of Sorangium cellulosum reveals unusual mechanisms of polyketide biosynthesis.
|
| |
Chem Biol,
13,
1277-1286.
|
 |
|
|
|
|
 |
M.Markova,
C.Peneff,
M.J.Hewlins,
T.Schirmer,
and
R.A.John
(2005).
Determinants of substrate specificity in omega-aminotransferases.
|
| |
J Biol Chem,
280,
36409-36416.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

