 |
PDBsum entry 1pv2

|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|
 241 a.a.
241 a.a.
|
 |

|

|

|

|

|

|

|


 257 a.a.
257 a.a.
|
 |

|

|

|

|

|

|

|



 270 a.a.
270 a.a.
|
 |

|

|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Chaperone
|
 |
|
Title:
|
 |
Native form 2 e.Coli chaperone hsp31
|
|
Structure:
|
 |
Chaperone protein hcha. Chain: a, b, c, d, e, f, g, h. Synonym: hsp31. Engineered: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Gene: hcha or b1967. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Biol. unit:
|
 |
 Dimer (from
)
Dimer (from
)
|
|
Resolution:
|
 |
|
2.71Å
|
R-factor:
|
0.225
|
R-free:
|
0.286
|
|
|
Authors:
|
 |
P.M.Quigley,K.Korotkov,F.Baneyx,W.G.J.Hol
|
Key ref:

|
 |
P.M.Quigley
et al.
(2004).
A new native EcHsp31 structure suggests a key role of structural flexibility for chaperone function.
Protein Sci,
13,
269-277.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
26-Jun-03
|
Release date:
|
13-Jan-04
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P31658
(HCHA_ECOLI) -
Protein/nucleic acid deglycase 1 from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
283 a.a.
241 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
Chains A, B, C, D, E, F, G, H:
E.C.3.5.1.124
- protein deglycase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
N(omega)-(1-hydroxy-2-oxopropyl)-L-arginyl-[protein] + H2O = lactate + L-arginyl-[protein] + H+
|
|
2.
|
N6-(1-hydroxy-2-oxopropyl)-L-lysyl-[protein] + H2O = lactate + L-lysyl-[protein] + H+
|
|
3.
|
S-(1-hydroxy-2-oxopropyl)-L-cysteinyl-[protein] + H2O = lactate + L-cysteinyl-[protein] + H+
|
|
 |
 |
 |
 |
 |
N(omega)-(1-hydroxy-2-oxopropyl)-L-arginyl-[protein]
|
+
|
H2O
|
=
|
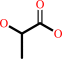 lactate
lactate
|
+
|
L-arginyl-[protein]
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
N(6)-(1-hydroxy-2-oxopropyl)-L-lysyl-[protein]
|
+
|
H2O
|
=
|
 lactate
lactate
|
+
|
L-lysyl-[protein]
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
S-(1-hydroxy-2-oxopropyl)-L-cysteinyl-[protein]
|
+
|
H2O
|
=
|
 lactate
lactate
|
+
|
L-cysteinyl-[protein]
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B, C, D, E, F, G, H:
E.C.4.2.1.130
- D-lactate dehydratase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
methylglyoxal + H2O = (R)-lactate + H+
|
 |
 |
 |
 |
 |
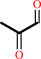 methylglyoxal
methylglyoxal
|
+
|
H2O
|
=
|
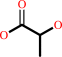 (R)-lactate
(R)-lactate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Protein Sci
13:269-277
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
A new native EcHsp31 structure suggests a key role of structural flexibility for chaperone function.
|
|
P.M.Quigley,
K.Korotkov,
F.Baneyx,
W.G.Hol.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Heat shock proteins and proteases play a crucial role in cell survival under
conditions of environmental stress. The heat shock protein Hsp31, produced by
gene hchA at elevated temperatures in Escherichia coli, is a homodimeric protein
consisting of a large A domain and a smaller P domain connected by a linker. Two
catalytic triads are present per dimer, with the Cys and His contributed by the
A domain and an Asp by the P domain. A new crystal Form II confirms the dimer
and catalytic triad arrangement seen in the earlier crystal Form I. In addition,
several loops exhibit increased flexibility compared to the previous Hsp31 dimer
structure. In particular, loops D2 and D3 are intriguing because their mobility
leads to the exposure of a sizable hydrophobic patch made up by surface areas of
both subunits near the dimer interface. The residues creating this hydrophobic
surface are completely conserved in the Hsp31 family. At the same time, access
to the catalytic triad is increased. These observations lead to the hypothesis
for the functioning of Hsp31 wherein loops D2 and D3 play a key role: first, at
elevated temperatures, by becoming mobile and uncovering a large hydrophobic
area that helps in binding to client proteins, and second, by removing the
client protein from the hydrophobic patch when the temperature decreases and the
loops adopt their low-temperature positions at the Hsp31 surface. The proposed
mode of action of flexible loops in the functioning of Hsp31 may be a general
principle employed by other chaperones.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Comparison of Form I EcHsp31 (top) and Form II
EcHsp31 dimer (bottom). Molecular surface of dimers shown with
hydrophobic patches in green. Ribbon representation of dimers
are shown on the right in the same orientation. Red ribbons in
Form I EcHsp31 highlight regions that are disordered in the Form
II EcHsp31. Note that the differences (red) between Form I
EcHsp31 and Form II EcHsp31 dimer result in the exposure of more
hydrophobic regions (green patches) in Form II EcHsp31. These
areas are circled in black.
|
 |
Figure 4.
Figure 4. Molecular surface of Form II EcHsp31 with the
linker region from Form I EcHsp31 superimposed to show how Tyr
29 limits access to the active site pocket. The surface of Form
II EcHsp31 is colored by domain--A (blue), and P (green), and
Cys 185 (yellow). Regions D2 and D3 are modeled in red and
orange ball and stick representation from their relative
position Form I EcHsp31 structure. Close-up view of the access
to Cys 185 of the triad is shown for both Form I and Form II.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the Protein Society:
Protein Sci
(2004,
13,
269-277)
copyright 2004.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.H.Fong,
B.A.Shoemaker,
S.O.Garbuzynskiy,
M.Y.Lobanov,
O.V.Galzitskaya,
and
A.R.Panchenko
(2009).
Intrinsic disorder in protein interactions: insights from a comprehensive structural analysis.
|
| |
PLoS Comput Biol,
5,
e1000316.
|
 |
|
|
|
|
 |
M.S.Sastry,
W.Zhou,
and
F.Baneyx
(2009).
Integrity of N- and C-termini is important for E. coli Hsp31 chaperone activity.
|
| |
Protein Sci,
18,
1439-1447.
|
 |
|
|
|
|
 |
W.Li,
J.Ju,
S.R.Rajski,
H.Osada,
and
B.Shen
(2008).
Characterization of the tautomycin biosynthetic gene cluster from Streptomyces spiroverticillatus unveiling new insights into dialkylmaleic anhydride and polyketide biosynthesis.
|
| |
J Biol Chem,
283,
28607-28617.
|
 |
|
|
|
|
 |
K.S.Sandhu,
and
D.Dash
(2007).
Dynamic alpha-helices: conformations that do not conform.
|
| |
Proteins,
68,
109-122.
|
 |
|
|
|
|
 |
K.S.Sandhu,
and
D.Dash
(2006).
Conformational flexibility may explain multiple cellular roles of PEST motifs.
|
| |
Proteins,
63,
727-732.
|
 |
|
|
|
|
 |
M.Mujacic,
and
F.Baneyx
(2006).
Regulation of Escherichia coli hchA, a stress-inducible gene encoding molecular chaperone Hsp31.
|
| |
Mol Microbiol,
60,
1576-1589.
|
 |
|
|
|
|
 |
F.Baneyx,
and
M.Mujacic
(2004).
Recombinant protein folding and misfolding in Escherichia coli.
|
| |
Nat Biotechnol,
22,
1399-1408.
|
 |
|
|
|
|
 |
M.S.Sastry,
P.M.Quigley,
W.G.Hol,
and
F.Baneyx
(2004).
The linker-loop region of Escherichia coli chaperone Hsp31 functions as a gate that modulates high-affinity substrate binding at elevated temperatures.
|
| |
Proc Natl Acad Sci U S A,
101,
8587-8592.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |
| |

