 |
PDBsum entry 1mo2

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.3.1.94
- 6-deoxyerythronolide-B synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
6 (S)-methylmalonyl-CoA + propanoyl-CoA + 6 NADPH + 12 H+ = 6-deoxyerythronolide B + 6 CO2 + 6 NADP+ + 7 CoA + H2O
|
 |
 |
 |
 |
 |
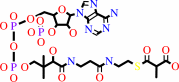 6
×
(S)-methylmalonyl-CoA
6
×
(S)-methylmalonyl-CoA
|
+
|
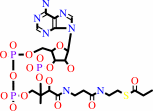 propanoyl-CoA
propanoyl-CoA
|
+
|
 6
×
NADPH
6
×
NADPH
|
+
|
12
×
H(+)
|
=
|
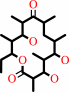 6-deoxyerythronolide B
6-deoxyerythronolide B
|
+
|
 6
×
CO2
6
×
CO2
|
+
|
 6
×
NADP(+)
6
×
NADP(+)
|
+
|
 7
×
CoA
7
×
CoA
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
41:12598-12606
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Insights into channel architecture and substrate specificity from crystal structures of two macrocycle-forming thioesterases of modular polyketide synthases.
|
|
S.C.Tsai,
H.Lu,
D.E.Cane,
C.Khosla,
R.M.Stroud.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Modular polyketide synthases (PKSs) synthesize the polyketide cores of
pharmacologically important natural products such as erythromycin and
picromycin. Understanding PKSs at high resolution could present new
opportunities for chemoenzymatic synthesis of complex molecules. The crystal
structures of macrocycle-forming thioesterase (TE) domains from the picromycin
synthase (PICS) and 6-deoxyerythronolide B synthase (DEBS) were determined to
1.8-3.0 A with an R(crys) of 19.2-24.4%, including three structures of PICS TE
(crystallized at pH 7.6, 8.0, and 8.4) and a second crystal form of DEBS TE. As
predicted by the previous work on DEBS TE [Tsai, S. C., et al. (2001) Proc.
Natl. Acad. Sci. U.S.A. 98, 14808-14813], PICS TE contains an open substrate
channel and a hydrophobic dimer interface. Notwithstanding their similarity, the
dimer interfaces and substrate channels of DEBS TE and PICS TE reveal key
differences. The structural basis for the divergent substrate specificities of
DEBS TE and PICS TE is analyzed. The size of the substrate channel increases
with increasing pH, presumably due to electrostatic repulsion in the channel at
elevated pH. Together, these structures support previous predictions that
macrocycle-forming thioesterases from PKSs share the same protein fold, an open
substrate channel, a similar catalytic mechanism, and a hydrophobic dimer
interface. They also provide a basis for the design of enzymes capable of
catalyzing regioselective macrocyclization of natural or synthetic substrates. A
series of high-resolution snapshots of a protein channel at different pHs is
presented alongside analysis of channel residues, which could help in the
redesign of the protein channel architecture.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.C.Cantu,
Y.Chen,
and
P.J.Reilly
(2010).
Thioesterases: a new perspective based on their primary and tertiary structures.
|
| |
Protein Sci,
19,
1281-1295.
|
 |
|
|
|
|
 |
D.L.Akey,
J.R.Razelun,
J.Tehranisa,
D.H.Sherman,
W.H.Gerwick,
and
J.L.Smith
(2010).
Crystal structures of dehydratase domains from the curacin polyketide biosynthetic pathway.
|
| |
Structure,
18,
94.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Buntin,
K.J.Weissman,
and
R.Müller
(2010).
An unusual thioesterase promotes isochromanone ring formation in ajudazol biosynthesis.
|
| |
Chembiochem,
11,
1137-1146.
|
 |
|
|
|
|
 |
L.Du,
and
L.Lou
(2010).
PKS and NRPS release mechanisms.
|
| |
Nat Prod Rep,
27,
255-278.
|
 |
|
|
|
|
 |
L.Tran,
R.W.Broadhurst,
M.Tosin,
A.Cavalli,
and
K.J.Weissman
(2010).
Insights into protein-protein and enzyme-substrate interactions in modular polyketide synthases.
|
| |
Chem Biol,
17,
705-716.
|
 |
|
|
|
|
 |
T.P.Korman,
J.M.Crawford,
J.W.Labonte,
A.G.Newman,
J.Wong,
C.A.Townsend,
and
S.C.Tsai
(2010).
Structure and function of an iterative polyketide synthase thioesterase domain catalyzing Claisen cyclization in aflatoxin biosynthesis.
|
| |
Proc Natl Acad Sci U S A,
107,
6246-6251.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Koglin,
and
C.T.Walsh
(2009).
Structural insights into nonribosomal peptide enzymatic assembly lines.
|
| |
Nat Prod Rep,
26,
987.
|
 |
|
|
|
|
 |
H.B.Claxton,
D.L.Akey,
M.K.Silver,
S.J.Admiraal,
and
J.L.Smith
(2009).
Structure and Functional Analysis of RifR, the Type II Thioesterase from the Rifamycin Biosynthetic Pathway.
|
| |
J Biol Chem,
284,
5021-5029.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.D.Kittendorf,
and
D.H.Sherman
(2009).
The methymycin/pikromycin pathway: a model for metabolic diversity in natural product biosynthesis.
|
| |
Bioorg Med Chem,
17,
2137-2146.
|
 |
|
|
|
|
 |
J.L.Meier,
and
M.D.Burkart
(2009).
The chemical biology of modular biosynthetic enzymes.
|
| |
Chem Soc Rev,
38,
2012-2045.
|
 |
|
|
|
|
 |
M.Kotowska,
K.Pawlik,
A.Smulczyk-Krawczyszyn,
H.Bartosz-Bechowski,
and
K.Kuczek
(2009).
Type II thioesterase ScoT, associated with Streptomyces coelicolor A3(2) modular polyketide synthase Cpk, hydrolyzes acyl residues and has a preference for propionate.
|
| |
Appl Environ Microbiol,
75,
887-896.
|
 |
|
|
|
|
 |
S.C.Tsai,
and
B.D.Ames
(2009).
Structural enzymology of polyketide synthases.
|
| |
Methods Enzymol,
459,
17-47.
|
 |
|
|
|
|
 |
H.Zhou,
J.Zhan,
K.Watanabe,
X.Xie,
and
Y.Tang
(2008).
A polyketide macrolactone synthase from the filamentous fungus Gibberella zeae.
|
| |
Proc Natl Acad Sci U S A,
105,
6249-6254.
|
 |
|
|
|
|
 |
J.L.Meier,
T.Barrows-Yano,
T.L.Foley,
C.L.Wike,
and
M.D.Burkart
(2008).
The unusual macrocycle forming thioesterase of mycolactone.
|
| |
Mol Biosyst,
4,
663-671.
|
 |
|
|
|
|
 |
K.J.Weissman,
and
R.Müller
(2008).
Protein-protein interactions in multienzyme megasynthetases.
|
| |
Chembiochem,
9,
826-848.
|
 |
|
|
|
|
 |
L.Betancor,
M.J.Fernández,
K.J.Weissman,
and
P.F.Leadlay
(2008).
Improved catalytic activity of a purified multienzyme from a modular polyketide synthase after coexpression with Streptomyces chaperonins in Escherichia coli.
|
| |
Chembiochem,
9,
2962-2966.
|
 |
|
|
|
|
 |
S.C.Wenzel,
H.B.Bode,
I.Kochems,
and
R.Müller
(2008).
A type I/type III polyketide synthase hybrid biosynthetic pathway for the structurally unique ansa compound kendomycin.
|
| |
Chembiochem,
9,
2711-2721.
|
 |
|
|
|
|
 |
T.Liu,
X.Lin,
X.Zhou,
Z.Deng,
and
D.E.Cane
(2008).
Mechanism of thioesterase-catalyzed chain release in the biosynthesis of the polyether antibiotic nanchangmycin.
|
| |
Chem Biol,
15,
449-458.
|
 |
|
|
|
|
 |
C.Khosla,
Y.Tang,
A.Y.Chen,
N.A.Schnarr,
and
D.E.Cane
(2007).
Structure and mechanism of the 6-deoxyerythronolide B synthase.
|
| |
Annu Rev Biochem,
76,
195-221.
|
 |
|
|
|
|
 |
F.Kopp,
and
M.A.Marahiel
(2007).
Macrocyclization strategies in polyketide and nonribosomal peptide biosynthesis.
|
| |
Nat Prod Rep,
24,
735-749.
|
 |
|
|
|
|
 |
N.Kadi,
D.Oves-Costales,
F.Barona-Gomez,
and
G.L.Challis
(2007).
A new family of ATP-dependent oligomerization-macrocyclization biocatalysts.
|
| |
Nat Chem Biol,
3,
652-656.
|
 |
|
|
|
|
 |
S.M.Ma,
and
Y.Tang
(2007).
Biochemical characterization of the minimal polyketide synthase domains in the lovastatin nonaketide synthase LovB.
|
| |
FEBS J,
274,
2854-2864.
|
 |
|
|
|
|
 |
S.Smith,
and
S.C.Tsai
(2007).
The type I fatty acid and polyketide synthases: a tale of two megasynthases.
|
| |
Nat Prod Rep,
24,
1041-1072.
|
 |
|
|
|
|
 |
A.M.Hill
(2006).
The biosynthesis, molecular genetics and enzymology of the polyketide-derived metabolites.
|
| |
Nat Prod Rep,
23,
256-320.
|
 |
|
|
|
|
 |
B.M.Harvey,
H.Hong,
M.A.Jones,
Z.A.Hughes-Thomas,
R.M.Goss,
M.L.Heathcote,
V.M.Bolanos-Garcia,
W.Kroutil,
J.Staunton,
P.F.Leadlay,
and
J.B.Spencer
(2006).
Evidence that a novel thioesterase is responsible for polyketide chain release during biosynthesis of the polyether ionophore monensin.
|
| |
Chembiochem,
7,
1435-1442.
|
 |
|
|
|
|
 |
D.L.Akey,
J.D.Kittendorf,
J.W.Giraldes,
R.A.Fecik,
D.H.Sherman,
and
J.L.Smith
(2006).
Structural basis for macrolactonization by the pikromycin thioesterase.
|
| |
Nat Chem Biol,
2,
537-542.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.D.Kittendorf,
and
D.H.Sherman
(2006).
Developing tools for engineering hybrid polyketide synthetic pathways.
|
| |
Curr Opin Biotechnol,
17,
597-605.
|
 |
|
|
|
|
 |
J.W.Giraldes,
D.L.Akey,
J.D.Kittendorf,
D.H.Sherman,
J.L.Smith,
and
R.A.Fecik
(2006).
Structural and mechanistic insights into polyketide macrolactonization from polyketide-based affinity labels.
|
| |
Nat Chem Biol,
2,
531-536.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Ghatge,
N.Palaniappan,
S.Das Choudhuri,
and
K.Reynolds
(2006).
Genetic manipulation of the biosynthetic process leading to phoslactomycins, potent protein phosphatase 2A inhibitors.
|
| |
J Ind Microbiol Biotechnol,
33,
589-599.
|
 |
|
|
|
|
 |
T.H.Davis,
and
R.M.Stroud
(2006).
Profile of Robert M. Stroud.
|
| |
Proc Natl Acad Sci U S A,
103,
5256-5258.
|
 |
|
|
|
|
 |
K.J.Weissman,
and
P.F.Leadlay
(2005).
Combinatorial biosynthesis of reduced polyketides.
|
| |
Nat Rev Microbiol,
3,
925-936.
|
 |
|
|
|
|
 |
C.D.Reeves
(2003).
The enzymology of combinatorial biosynthesis.
|
| |
Crit Rev Biotechnol,
23,
95.
|
 |
|
|
|
|
 |
K.Watanabe,
C.C.Wang,
C.N.Boddy,
D.E.Cane,
and
C.Khosla
(2003).
Understanding substrate specificity of polyketide synthase modules by generating hybrid multimodular synthases.
|
| |
J Biol Chem,
278,
42020-42026.
|
 |
|
|
|
|
 |
N.Palaniappan,
B.S.Kim,
Y.Sekiyama,
H.Osada,
and
K.A.Reynolds
(2003).
Enhancement and selective production of phoslactomycin B, a protein phosphatase IIa inhibitor, through identification and engineering of the corresponding biosynthetic gene cluster.
|
| |
J Biol Chem,
278,
35552-35557.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

