 |
PDBsum entry 1joa

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1joa
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.11.1.1
- Nadh peroxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
H2O2 + NADH + H+ = NAD+ + 2 H2O
|
 |
 |
 |
 |
 |
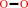 H2O2
H2O2
|
+
|
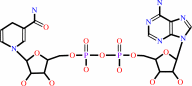 NADH
NADH
|
+
|
H(+)
|
=
|
 NAD(+)
NAD(+)
|
+
|
2
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
35:9951-9957
(1996)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the native cysteine-sulfenic acid redox center of enterococcal NADH peroxidase refined at 2.8 A resolution.
|
|
J.I.Yeh,
A.Claiborne,
W.G.Hol.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
In order to obtain the crystal structure of the flavoprotein NADH peroxidase
with its native Cys42-sulfenic acid redox center, a strategy combining reduced
exposure of crystals to ambient oxygen and data collection at -160 degrees C was
applied. The structure of the native enzyme to 2.8 A resolution is described;
these results conclusively establish the existence of the Cys42-sulfenic acid as
the functional non-flavin redox center of the peroxidase and provide the first
structure for any naturally occurring protein-sulfenic acid. The Cys42-sulfenic
acid atoms C alpha-C beta-S gamma-O roughly define a planar arrangement which is
stacked parallel to the si face of the FAD isoalloxazine and positions the
sulfenyl oxygen atom only 3.3 A from FAD-C4A. His10-N epsilon 2 contributes a
hydrogen bond to the sulfenic acid oxygen, at a distance of 3.2 A. Although one
oxygen atom (OX1) of the non-native Cys42-sulfonic acid derivative identified in
the earlier wild-type peroxidase structure was taken to represent the native
Cys42-sulfenic acid oxygen [Stehle, T., Ahmed, S. A., Claiborne, A., &
Schulz, G. E. (1991) J. Mol. Biol. 221, 1325-1344], this structure shows that
the sulfenic acid oxygen does not occupy this position, nor is it
hydrogen-bonded to Cys42-N as was OX1. Comparison of the native Cys42-sulfenic
acid structure with that of two-electron reduced glutathione reductase provides
an insight into the sulfenic acid FAD charge-transfer interaction observed with
both wild-type and His10 mutant peroxidases. A model of the E.NADH intermediate
recently observed in stopped-flow analyses of the enzyme [Crane, E. J., III,
Parsonage, D., Poole, L. B., & Claiborne, A. (1995) Biochemistry 34,
14114-14124] has also been generated to assist in analyzing the chemical
mechanism of sulfenic acid reduction.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.S.Rehder,
and
C.R.Borges
(2010).
Possibilities and pitfalls in quantifying the extent of cysteine sulfenic acid modification of specific proteins within complex biofluids.
|
| |
BMC Biochem,
11,
25.
|
 |
|
|
|
|
 |
F.R.Salsbury,
S.T.Knutson,
L.B.Poole,
and
J.S.Fetrow
(2008).
Functional site profiling and electrostatic analysis of cysteines modifiable to cysteine sulfenic acid.
|
| |
Protein Sci,
17,
299-312.
|
 |
|
|
|
|
 |
G.Wang,
C.Strang,
P.J.Pfaffinger,
and
M.Covarrubias
(2007).
Zn2+-dependent redox switch in the intracellular T1-T1 interface of a Kv channel.
|
| |
J Biol Chem,
282,
13637-13647.
|
 |
|
|
|
|
 |
H.Shi,
T.Xia,
A.E.Nel,
and
J.I.Yeh
(2007).
Part II: coordinated biosensors--development of enhanced nanobiosensors for biological and medical applications.
|
| |
Nanomed,
2,
599-614.
|
 |
|
|
|
|
 |
V.Shetty,
D.S.Spellman,
and
T.A.Neubert
(2007).
Characterization by tandem mass spectrometry of stable cysteine sulfenic acid in a cysteine switch peptide of matrix metalloproteinases.
|
| |
J Am Soc Mass Spectrom,
18,
1544-1551.
|
 |
|
|
|
|
 |
J.R.Stone,
and
S.Yang
(2006).
Hydrogen peroxide: a signaling messenger.
|
| |
Antioxid Redox Signal,
8,
243-270.
|
 |
|
|
|
|
 |
J.I.Yeh,
M.B.Zimmt,
and
A.L.Zimmerman
(2005).
Nanowiring of a redox enzyme by metallized peptides.
|
| |
Biosens Bioelectron,
21,
973-978.
|
 |
|
|
|
|
 |
G.T.Lountos,
B.R.Riebel,
W.B.Wellborn,
A.S.Bommarius,
and
A.M.Orville
(2004).
Crystallization and preliminary analysis of a water-forming NADH oxidase from Lactobacillus sanfranciscensis.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
2044-2047.
|
 |
|
|
|
|
 |
K.S.Yang,
S.W.Kang,
H.A.Woo,
S.C.Hwang,
H.Z.Chae,
K.Kim,
and
S.G.Rhee
(2002).
Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid.
|
| |
J Biol Chem,
277,
38029-38036.
|
 |
|
|
|
|
 |
R.J.Mallis,
M.J.Hamann,
W.Zhao,
T.Zhang,
S.Hendrich,
and
J.A.Thomas
(2002).
Irreversible thiol oxidation in carbonic anhydrase III: protection by S-glutathiolation and detection in aging rats.
|
| |
Biol Chem,
383,
649-662.
|
 |
|
|
|
|
 |
A.Changela,
C.K.Ho,
A.Martins,
S.Shuman,
and
A.Mondragón
(2001).
Structure and mechanism of the RNA triphosphatase component of mammalian mRNA capping enzyme.
|
| |
EMBO J,
20,
2575-2586.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.T.Hilgers,
and
M.L.Ludwig
(2001).
Crystal structure of the quorum-sensing protein LuxS reveals a catalytic metal site.
|
| |
Proc Natl Acad Sci U S A,
98,
11169-11174.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
O.Dym,
and
D.Eisenberg
(2001).
Sequence-structure analysis of FAD-containing proteins.
|
| |
Protein Sci,
10,
1712-1728.
|
 |
|
|
|
|
 |
E.J.Crane,
J.I.Yeh,
J.Luba,
and
A.Claiborne
(2000).
Analysis of the kinetic and redox properties of the NADH peroxidase R303M mutant: correlation with the crystal structure.
|
| |
Biochemistry,
39,
10353-10364.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Y.King,
J.A.Horenstein,
and
M.G.Caparon
(2000).
Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes.
|
| |
J Bacteriol,
182,
5290-5299.
|
 |
|
|
|
|
 |
N.M.Okeley,
and
W.A.van der Donk
(2000).
Novel cofactors via post-translational modifications of enzyme active sites.
|
| |
Chem Biol,
7,
R159-R171.
|
 |
|
|
|
|
 |
A.Claiborne,
J.I.Yeh,
T.C.Mallett,
J.Luba,
E.J.Crane,
V.Charrier,
and
D.Parsonage
(1999).
Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation.
|
| |
Biochemistry,
38,
15407-15416.
|
 |
|
|
|
|
 |
I.Endo,
M.Odaka,
and
M.Yohda
(1999).
An enzyme controlled by light: the molecular mechanism of photoreactivity in nitrile hydratase.
|
| |
Trends Biotechnol,
17,
244-248.
|
 |
|
|
|
|
 |
J.Luba,
V.Charrier,
and
A.Claiborne
(1999).
Coenzyme A-disulfide reductase from Staphylococcus aureus: evidence for asymmetric behavior on interaction with pyridine nucleotides.
|
| |
Biochemistry,
38,
2725-2737.
|
 |
|
|
|
|
 |
T.C.Mallett,
D.Parsonage,
and
A.Claiborne
(1999).
Equilibrium analyses of the active-site asymmetry in enterococcal NADH oxidase: role of the cysteine-sulfenic acid redox center.
|
| |
Biochemistry,
38,
3000-3011.
|
 |
|
|
|
|
 |
T.M.Iverson,
C.Luna-Chavez,
G.Cecchini,
and
D.C.Rees
(1999).
Structure of the Escherichia coli fumarate reductase respiratory complex.
|
| |
Science,
284,
1961-1966.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Caselli,
R.Marzocchini,
G.Camici,
G.Manao,
G.Moneti,
G.Pieraccini,
and
G.Ramponi
(1998).
The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2.
|
| |
J Biol Chem,
273,
32554-32560.
|
 |
|
|
|
|
 |
H.J.Choi,
S.W.Kang,
C.H.Yang,
S.G.Rhee,
and
S.E.Ryu
(1998).
Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution.
|
| |
Nat Struct Biol,
5,
400-406.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.P.Fierobe,
E.Mirgorodskaya,
K.A.McGuire,
P.Roepstorff,
B.Svensson,
and
A.J.Clarke
(1998).
Restoration of catalytic activity beyond wild-type level in glucoamylase from Aspergillus awamori by oxidation of the Glu400-->Cys catalytic-base mutant to cysteinesulfinic acid.
|
| |
Biochemistry,
37,
3743-3752.
|
 |
|
|
|
|
 |
J.S.Stamler,
and
A.Hausladen
(1998).
Oxidative modifications in nitrosative stress.
|
| |
Nat Struct Biol,
5,
247-249.
|
 |
|
|
|
|
 |
K.Becker,
S.N.Savvides,
M.Keese,
R.H.Schirmer,
and
P.A.Karplus
(1998).
Enzyme inactivation through sulfhydryl oxidation by physiologic NO-carriers.
|
| |
Nat Struct Biol,
5,
267-271.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Mewies,
W.S.McIntire,
and
N.S.Scrutton
(1998).
Covalent attachment of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) to enzymes: the current state of affairs.
|
| |
Protein Sci,
7,
7.
|
 |
|
|
|
|
 |
S.B.delCardayre,
and
J.E.Davies
(1998).
Staphylococcus aureus coenzyme A disulfide reductase, a new subfamily of pyridine nucleotide-disulfide oxidoreductase. Sequence, expression, and analysis of cdr.
|
| |
J Biol Chem,
273,
5752-5757.
|
 |
|
|
|
|
 |
S.Nagashima,
M.Nakasako,
N.Dohmae,
M.Tsujimura,
K.Takio,
M.Odaka,
M.Yohda,
N.Kamiya,
and
I.Endo
(1998).
Novel non-heme iron center of nitrile hydratase with a claw setting of oxygen atoms.
|
| |
Nat Struct Biol,
5,
347-351.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.W.Kang,
I.C.Baines,
and
S.G.Rhee
(1998).
Characterization of a mammalian peroxiredoxin that contains one conserved cysteine.
|
| |
J Biol Chem,
273,
6303-6311.
|
 |
|
|
|
|
 |
T.C.Mallett,
and
A.Claiborne
(1998).
Oxygen reactivity of an NADH oxidase C42S mutant: evidence for a C(4a)-peroxyflavin intermediate and a rate-limiting conformational change.
|
| |
Biochemistry,
37,
8790-8802.
|
 |
|
|
|
|
 |
E.J.Crane,
J.Vervoort,
and
A.Claiborne
(1997).
13C NMR analysis of the cysteine-sulfenic acid redox center of enterococcal NADH peroxidase.
|
| |
Biochemistry,
36,
8611-8618.
|
 |
|
|
|
|
 |
H.R.Ellis,
and
L.B.Poole
(1997).
Roles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium.
|
| |
Biochemistry,
36,
13349-13356.
|
 |
|
|
|
|
 |
H.R.Ellis,
and
L.B.Poole
(1997).
Novel application of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase.
|
| |
Biochemistry,
36,
15013-15018.
|
 |
|
|
|
|
 |
M.Li Calzi,
and
L.B.Poole
(1997).
Requirement for the two AhpF cystine disulfide centers in catalysis of peroxide reduction by alkyl hydroperoxide reductase.
|
| |
Biochemistry,
36,
13357-13364.
|
 |
|
|
|
|
 |
M.Tsujimura,
N.Dohmae,
M.Odaka,
M.Chijimatsu,
K.Takio,
M.Yohda,
M.Hoshino,
S.Nagashima,
and
I.Endo
(1997).
Structure of the photoreactive iron center of the nitrile hydratase from Rhodococcus sp. N-771. Evidence of a novel post-translational modification in the cysteine ligand.
|
| |
J Biol Chem,
272,
29454-29459.
|
 |
|
|
|
|
 |
W.S.Willett,
and
S.D.Copley
(1996).
Identification and localization of a stable sulfenic acid in peroxide-treated tetrachlorohydroquinone dehalogenase using electrospray mass spectrometry.
|
| |
Chem Biol,
3,
851-857.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

