 |
PDBsum entry 1cs1

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.5.1.48
- cystathionine gamma-synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
O-succinyl-L-homoserine + L-cysteine = L,L-cystathionine + succinate + H+
|
 |
 |
 |
 |
 |
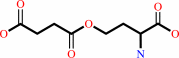 O-succinyl-L-homoserine
O-succinyl-L-homoserine
|
+
|
 L-cysteine
L-cysteine
|
=
|
L,L-cystathionine
|
+
|
 succinate
succinate
|
+
|
H(+)
Bound ligand (Het Group name = )
matches with 58.33% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
 Pyridoxal 5'-phosphate
Pyridoxal 5'-phosphate
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Embo J
17:6827-6838
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of Escherichia coli cystathionine gamma-synthase at 1.5 A resolution.
|
|
T.Clausen,
R.Huber,
L.Prade,
M.C.Wahl,
A.Messerschmidt.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The transsulfuration enzyme cystathionine gamma-synthase (CGS) catalyses the
pyridoxal 5'-phosphate (PLP)-dependent gamma-replacement of
O-succinyl-L-homoserine and L-cysteine, yielding L-cystathionine. The crystal
structure of the Escherichia coli enzyme has been solved by molecular
replacement with the known structure of cystathionine beta-lyase (CBL), and
refined at 1.5 A resolution to a crystallographic R-factor of 20.0%. The enzyme
crystallizes as an alpha4 tetramer with the subunits related by
non-crystallographic 222 symmetry. The spatial fold of the subunits, with three
functionally distinct domains and their quaternary arrangement, is similar to
that of CBL. Previously proposed reaction mechanisms for CGS can be checked
against the structural model, allowing interpretation of the catalytic and
substrate-binding functions of individual active site residues. Enzyme-substrate
models pinpoint specific residues responsible for the substrate specificity, in
agreement with structural comparisons with CBL. Both steric and electrostatic
designs of the active site seem to achieve proper substrate selection and
productive orientation. Amino acid sequence and structural alignments of CGS and
CBL suggest that differences in the substrate-binding characteristics are
responsible for the different reaction chemistries. Because CGS catalyses the
only known PLP-dependent replacement reaction at Cgamma of certain amino acids,
the results will help in our understanding of the chemical versatility of PLP.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.S.El-Sayed
(2010).
Microbial L-methioninase: production, molecular characterization, and therapeutic applications.
|
| |
Appl Microbiol Biotechnol,
86,
445-467.
|
 |
|
|
|
|
 |
P.H.Lodha,
A.F.Jaworski,
and
S.M.Aitken
(2010).
Characterization of site-directed mutants of residues R58, R59, D116, W340 and R372 in the active site of E. coli cystathionine beta-lyase.
|
| |
Protein Sci,
19,
383-391.
|
 |
|
|
|
|
 |
A.Farsi,
P.H.Lodha,
J.E.Skanes,
H.Los,
N.Kalidindi,
and
S.M.Aitken
(2009).
Interconversion of a pair of active-site residues in Escherichia coli cystathionine gamma-synthase, E. coli cystathionine beta-lyase, and Saccharomyces cerevisiae cystathionine gamma-lyase and development of tools for the investigation of their mechanisms and reaction specificity.
|
| |
Biochem Cell Biol,
87,
445-457.
|
 |
|
|
|
|
 |
P.Patel,
M.Vatish,
J.Heptinstall,
R.Wang,
and
R.J.Carson
(2009).
The endogenous production of hydrogen sulphide in intrauterine tissues.
|
| |
Reprod Biol Endocrinol,
7,
10.
|
 |
|
|
|
|
 |
Q.Sun,
R.Collins,
S.Huang,
L.Holmberg-Schiavone,
G.S.Anand,
C.H.Tan,
S.van-den-Berg,
L.W.Deng,
P.K.Moore,
T.Karlberg,
and
J.Sivaraman
(2009).
Structural Basis for the Inhibition Mechanism of Human Cystathionine {gamma}-Lyase, an Enzyme Responsible for the Production of H2S.
|
| |
J Biol Chem,
284,
3076-3085.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.Kudou,
S.Misaki,
M.Yamashita,
T.Tamura,
N.Esaki,
and
K.Inagaki
(2008).
The role of cysteine 116 in the active site of the antitumor enzyme L-methionine gamma-lyase from Pseudomonas putida.
|
| |
Biosci Biotechnol Biochem,
72,
1722-1730.
|
 |
|
|
|
|
 |
O.M.Ganichkin,
X.M.Xu,
B.A.Carlson,
H.Mix,
D.L.Hatfield,
V.N.Gladyshev,
and
M.C.Wahl
(2008).
Structure and catalytic mechanism of eukaryotic selenocysteine synthase.
|
| |
J Biol Chem,
283,
5849-5865.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
O.Cholet,
A.Hénaut,
and
P.Bonnarme
(2007).
Transcriptional analysis of L-methionine catabolism in Brevibacterium linens ATCC9175.
|
| |
Appl Microbiol Biotechnol,
74,
1320-1332.
|
 |
|
|
|
|
 |
K.Manikandan,
and
S.Ramakumar
(2004).
The occurrence of C--H...O hydrogen bonds in alpha-helices and helix termini in globular proteins.
|
| |
Proteins,
56,
768-781.
|
 |
|
|
|
|
 |
A.Messerschmidt,
M.Worbs,
C.Steegborn,
M.C.Wahl,
R.Huber,
B.Laber,
and
T.Clausen
(2003).
Determinants of enzymatic specificity in the Cys-Met-metabolism PLP-dependent enzymes family: crystal structure of cystathionine gamma-lyase from yeast and intrafamiliar structure comparison.
|
| |
Biol Chem,
384,
373-386.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.A.Carter,
P.S.Worsley,
G.Sawers,
G.L.Challis,
M.J.Dilworth,
K.C.Carson,
J.A.Lawrence,
M.Wexler,
A.W.Johnston,
and
K.H.Yeoman
(2002).
The vbs genes that direct synthesis of the siderophore vicibactin in Rhizobium leguminosarum: their expression in other genera requires ECF sigma factor RpoI.
|
| |
Mol Microbiol,
44,
1153-1166.
|
 |
|
|
|
|
 |
L.A.Nahum,
and
M.Riley
(2001).
Divergence of function in sequence-related groups of Escherichia coli proteins.
|
| |
Genome Res,
11,
1375-1381.
|
 |
|
|
|
|
 |
G.Schneider,
H.Käck,
and
Y.Lindqvist
(2000).
The manifold of vitamin B6 dependent enzymes.
|
| |
Structure,
8,
R1-R6.
|
 |
|
|
|
|
 |
H.Inoue,
K.Inagaki,
N.Adachi,
T.Tamura,
N.Esaki,
K.Soda,
and
H.Tanaka
(2000).
Role of tyrosine 114 of L-methionine gamma-lyase from Pseudomonas putida.
|
| |
Biosci Biotechnol Biochem,
64,
2336-2343.
|
 |
|
|
|
|
 |
C.Steegborn,
T.Clausen,
P.Sondermann,
U.Jacob,
M.Worbs,
S.Marinkovic,
R.Huber,
and
M.C.Wahl
(1999).
Kinetics and inhibition of recombinant human cystathionine gamma-lyase. Toward the rational control of transsulfuration.
|
| |
J Biol Chem,
274,
12675-12684.
|
 |
|
|
|
|
 |
S.Roy
(1999).
Multifunctional enzymes and evolution of biosynthetic pathways: retro-evolution by jumps.
|
| |
Proteins,
37,
303-309.
|
 |
|
|
|
|
 |
T.Clausen,
M.C.Wahl,
A.Messerschmidt,
R.Huber,
J.C.Fuhrmann,
B.Laber,
W.Streber,
and
C.Steegborn
(1999).
Cloning, purification and characterisation of cystathionine gamma-synthase from Nicotiana tabacum.
|
| |
Biol Chem,
380,
1237-1242.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

