 |
PDBsum entry 1bj4

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.1.2.1
- glycine hydroxymethyltransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Folate Coenzymes
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(6R)-5,10-methylene-5,6,7,8-tetrahydrofolate + glycine + H2O = (6S)- 5,6,7,8-tetrahydrofolate + L-serine
|
 |
 |
 |
 |
 |
(6R)-5,10-methylene-5,6,7,8-tetrahydrofolate
|
+
|
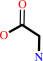 glycine
glycine
|
+
|
H2O
|
=
|
(6S)- 5,6,7,8-tetrahydrofolate
|
+
|
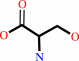 L-serine
L-serine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
6:1105-1116
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
The crystal structure of human cytosolic serine hydroxymethyltransferase: a target for cancer chemotherapy.
|
|
S.B.Renwick,
K.Snell,
U.Baumann.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
BACKGROUND: Serine hydroxymethyltransferase (SHMT) is a ubiquitous enzyme found
in all prokaryotes and eukaryotes. As an enzyme of the thymidylate synthase
metabolic cycle, SHMT catalyses the retro-aldol cleavage of serine to glycine,
with the resulting hydroxymethyl group being transferred to tetrahydrofolate to
form 5, 10-methylene-tetrahydrofolate. The latter is the major source of
one-carbon units in metabolism. Elevated SHMT activity has been shown to be
coupled to the increased demand for DNA synthesis in rapidly proliferating
cells, particularly tumour cells. Consequently, the central role of SHMT in
nucleotide biosynthesis makes it an attractive target for cancer chemotherapy.
RESULTS: We have solved the crystal structure of human cytosolic SHMT by
multiple isomorphous replacement to 2.65 A resolution. The monomer has a fold
typical for alpha class pyridoxal 5'-phosphate (PLP) dependent enzymes. The
tetramer association is best described as a 'dimer of dimers' where residues
from both subunits of one 'tight' dimer contribute to the active site.
CONCLUSIONS: The crystal structure shows the evolutionary relationship between
SHMT and other alpha class PLP-dependent enzymes, as the fold is highly
conserved. Many of the results of site-directed mutagenesis studies can easily
be rationalised or re-interpreted in light of the structure presented here. For
example, His 151 is not the catalytic base, contrary to the findings of others.
A mechanism for the cleavage of serine to glycine and formaldehyde is proposed.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |
Figure 7.
Figure 7. Steroview comparisons of the active sites from
different PLP-dependent enzymes. (a) Overlay of the active sites
of aspartate aminotransferase (AAT; cyan) and SHMT (green). (b)
Overlay of ornithine decarboxylase (ORD; cyan) and SHMT (green).
|
 |
|
|

|
| |
The above figure is
reprinted
by permission from Cell Press:
Structure
(1998,
6,
1105-1116)
copyright 1998.
|
|
| |
Figure was
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
F.Daidone,
R.Florio,
S.Rinaldo,
R.Contestabile,
M.L.di Salvo,
F.Cutruzzolà,
F.Bossa,
and
A.Paiardini
(2011).
In silico and in vitro validation of serine hydroxymethyltransferase as a chemotherapeutic target of the antifolate drug pemetrexed.
|
| |
Eur J Med Chem,
46,
1616-1621.
|
 |
|
|
|
|
 |
G.H.Zhao,
H.Li,
W.Liu,
W.G.Zhang,
F.Zhang,
Q.Liu,
and
Q.C.Jiao
(2011).
Preparation of optically active β-hydroxy-α-amino acid by immobilized Escherichia coli cells with serine hydroxymethyl transferase activity.
|
| |
Amino Acids,
40,
215-220.
|
 |
|
|
|
|
 |
M.Maekawa,
T.Ohnishi,
K.Hashimoto,
A.Watanabe,
Y.Iwayama,
H.Ohba,
E.Hattori,
K.Yamada,
and
T.Yoshikawa
(2010).
Analysis of strain-dependent prepulse inhibition points to a role for Shmt1 (SHMT1) in mice and in schizophrenia.
|
| |
J Neurochem,
115,
1374-1385.
|
 |
|
|
|
|
 |
C.K.Pang,
J.H.Hunter,
R.Gujjar,
R.Podutoori,
J.Bowman,
D.G.Mudeppa,
and
P.K.Rathod
(2009).
Catalytic and ligand-binding characteristics of Plasmodium falciparum serine hydroxymethyltransferase.
|
| |
Mol Biochem Parasitol,
168,
74-83.
|
 |
|
|
|
|
 |
R.Florio,
R.Chiaraluce,
V.Consalvi,
A.Paiardini,
B.Catacchio,
F.Bossa,
and
R.Contestabile
(2009).
The role of evolutionarily conserved hydrophobic contacts in the quaternary structure stability of Escherichia coli serine hydroxymethyltransferase.
|
| |
FEBS J,
276,
132-143.
|
 |
|
|
|
|
 |
S.Lima,
R.Khristoforov,
C.Momany,
and
R.S.Phillips
(2007).
Crystal structure of Homo sapiens kynureninase.
|
| |
Biochemistry,
46,
2735-2744.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.Rajaram,
B.S.Bhavani,
P.Kaul,
V.Prakash,
N.Appaji Rao,
H.S.Savithri,
and
M.R.Murthy
(2007).
Structure determination and biochemical studies on Bacillus stearothermophilus E53Q serine hydroxymethyltransferase and its complexes provide insights on function and enzyme memory.
|
| |
FEBS J,
274,
4148-4160.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Mukherjee,
S.A.Sievers,
M.T.Brown,
and
P.J.Johnson
(2006).
Identification and biochemical characterization of serine hydroxymethyl transferase in the hydrogenosome of Trichomonas vaginalis.
|
| |
Eukaryot Cell,
5,
2072-2078.
|
 |
|
|
|
|
 |
V.Schirch,
and
D.M.Szebenyi
(2005).
Serine hydroxymethyltransferase revisited.
|
| |
Curr Opin Chem Biol,
9,
482-487.
|
 |
|
|
|
|
 |
A.N.Bhatt,
M.Y.Khan,
and
V.Bhakuni
(2004).
The C-terminal domain of dimeric serine hydroxymethyltransferase plays a key role in stabilization of the quaternary structure and cooperative unfolding of protein: domain swapping studies with enzymes having high sequence identity.
|
| |
Protein Sci,
13,
2184-2195.
|
 |
|
|
|
|
 |
A.Paiardini,
F.Bossa,
and
S.Pascarella
(2004).
Evolutionarily conserved regions and hydrophobic contacts at the superfamily level: The case of the fold-type I, pyridoxal-5'-phosphate-dependent enzymes.
|
| |
Protein Sci,
13,
2992-3005.
|
 |
|
|
|
|
 |
A.Paiardini,
G.Gianese,
F.Bossa,
and
S.Pascarella
(2003).
Structural plasticity of thermophilic serine hydroxymethyltransferases.
|
| |
Proteins,
50,
122-134.
|
 |
|
|
|
|
 |
K.A.Zanetti,
and
P.J.Stover
(2003).
Pyridoxal phosphate inhibits dynamic subunit interchange among serine hydroxymethyltransferase tetramers.
|
| |
J Biol Chem,
278,
10142-10149.
|
 |
|
|
|
|
 |
S.Angelaccio,
R.Chiaraluce,
V.Consalvi,
B.Buchenau,
L.Giangiacomo,
F.Bossa,
and
R.Contestabile
(2003).
Catalytic and thermodynamic properties of tetrahydromethanopterin-dependent serine hydroxymethyltransferase from Methanococcus jannaschii.
|
| |
J Biol Chem,
278,
41789-41797.
|
 |
|
|
|
|
 |
S.Chaturvedi,
and
V.Bhakuni
(2003).
Unusual structural, functional, and stability properties of serine hydroxymethyltransferase from Mycobacterium tuberculosis.
|
| |
J Biol Chem,
278,
40793-40805.
|
 |
|
|
|
|
 |
T.F.Fu,
E.S.Boja,
M.K.Safo,
and
V.Schirch
(2003).
Role of proline residues in the folding of serine hydroxymethyltransferase.
|
| |
J Biol Chem,
278,
31088-31094.
|
 |
|
|
|
|
 |
T.F.Fu,
J.N.Scarsdale,
G.Kazanina,
V.Schirch,
and
H.T.Wright
(2003).
Location of the pteroylpolyglutamate-binding site on rabbit cytosolic serine hydroxymethyltransferase.
|
| |
J Biol Chem,
278,
2645-2653.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Y.Yang,
and
U.T.Meier
(2003).
Genetic interaction between a chaperone of small nucleolar ribonucleoprotein particles and cytosolic serine hydroxymethyltransferase.
|
| |
J Biol Chem,
278,
23553-23560.
|
 |
|
|
|
|
 |
V.R.Jala,
V.Prakash,
N.A.Rao,
and
H.S.Savithri
(2002).
Overexpression and characterization of dimeric and tetrameric forms of recombinant serine hydroxymethyltransferase from Bacillus stearothermophilus.
|
| |
J Biosci,
27,
233-242.
|
 |
|
|
|
|
 |
V.Trivedi,
A.Gupta,
V.R.Jala,
P.Saravanan,
G.S.Rao,
N.A.Rao,
H.S.Savithri,
and
H.S.Subramanya
(2002).
Crystal structure of binary and ternary complexes of serine hydroxymethyltransferase from Bacillus stearothermophilus: insights into the catalytic mechanism.
|
| |
J Biol Chem,
277,
17161-17169.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Soda,
T.Yoshimura,
and
N.Esaki
(2001).
Stereospecificity for the hydrogen transfer of pyridoxal enzyme reactions.
|
| |
Chem Rec,
1,
373-384.
|
 |
|
|
|
|
 |
R.Contestabile,
A.Paiardini,
S.Pascarella,
M.L.di Salvo,
S.D'Aguanno,
and
F.Bossa
(2001).
l-Threonine aldolase, serine hydroxymethyltransferase and fungal alanine racemase. A subgroup of strictly related enzymes specialized for different functions.
|
| |
Eur J Biochem,
268,
6508-6525.
|
 |
|
|
|
|
 |
D.M.Szebenyi,
X.Liu,
I.A.Kriksunov,
P.J.Stover,
and
D.J.Thiel
(2000).
Structure of a murine cytoplasmic serine hydroxymethyltransferase quinonoid ternary complex: evidence for asymmetric obligate dimers.
|
| |
Biochemistry,
39,
13313-13323.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Schneider,
H.Käck,
and
Y.Lindqvist
(2000).
The manifold of vitamin B6 dependent enzymes.
|
| |
Structure,
8,
R1-R6.
|
 |
|
|
|
|
 |
H.Ogawa,
T.Gomi,
and
M.Fujioka
(2000).
Serine hydroxymethyltransferase and threonine aldolase: are they identical?
|
| |
Int J Biochem Cell Biol,
32,
289-301.
|
 |
|
|
|
|
 |
J.V.Rao,
V.Prakash,
N.A.Rao,
and
H.S.Savithri
(2000).
The role of Glu74 and Tyr82 in the reaction catalyzed by sheep liver cytosolic serine hydroxymethyltransferase.
|
| |
Eur J Biochem,
267,
5967-5976.
|
 |
|
|
|
|
 |
N.A.Rao,
R.Talwar,
and
H.S.Savithri
(2000).
Molecular organization, catalytic mechanism and function of serine hydroxymethyltransferase--a potential target for cancer chemotherapy.
|
| |
Int J Biochem Cell Biol,
32,
405-416.
|
 |
|
|
|
|
 |
R.Contestabile,
S.Angelaccio,
F.Bossa,
H.T.Wright,
N.Scarsdale,
G.Kazanina,
and
V.Schirch
(2000).
Role of tyrosine 65 in the mechanism of serine hydroxymethyltransferase.
|
| |
Biochemistry,
39,
7492-7500.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Talwar,
J.R.Jagath,
N.A.Rao,
and
H.S.Savithri
(2000).
His230 of serine hydroxymethyltransferase facilitates the proton abstraction step in catalysis.
|
| |
Eur J Biochem,
267,
1441-1446.
|
 |
|
|
|
|
 |
R.Talwar,
N.A.Rao,
and
H.S.Savithri
(2000).
A change in reaction specificity of sheep liver serine hydroxymethyltransferase. Induction of NADH oxidation upon mutation of His230 to Tyr.
|
| |
Eur J Biochem,
267,
929-934.
|
 |
|
|
|
|
 |
T.Fujii,
M.Maeda,
H.Mihara,
T.Kurihara,
N.Esaki,
and
Y.Hata
(2000).
Structure of a NifS homologue: X-ray structure analysis of CsdB, an Escherichia coli counterpart of mammalian selenocysteine lyase.
|
| |
Biochemistry,
39,
1263-1273.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.N.Scarsdale,
G.Kazanina,
S.Radaev,
V.Schirch,
and
H.T.Wright
(1999).
Crystal structure of rabbit cytosolic serine hydroxymethyltransferase at 2.8 A resolution: mechanistic implications.
|
| |
Biochemistry,
38,
8347-8358.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.N.Jansonius
(1998).
Structure, evolution and action of vitamin B6-dependent enzymes.
|
| |
Curr Opin Struct Biol,
8,
759-769.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

