 |
PDBsum entry 7ljs
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Flavoprotein
|
 |
|
Title:
|
 |
Porcine dihydropyrimidine dehydrogenase (dpd) complexed with 5- ethynyluracil (5eu) - open form
|
|
Structure:
|
 |
Dihydropyrimidine dehydrogenase [nadp(+)]. Chain: a, b, c, d. Synonym: dpd,dihydrothymine dehydrogenase,dihydrouracil dehydrogenase. Engineered: yes
|
|
Source:
|
 |
Sus scrofa. Pig. Organism_taxid: 9823. Gene: dpyd. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.00Å
|
R-factor:
|
0.175
|
R-free:
|
0.229
|
|
|
Authors:
|
 |
A.Butrin,D.Forouzesh,B.Beaupre,Z.Wawrzak,D.Liu,G.Moran
|
|
Key ref:
|
 |
D.C.Forouzesh
et al.
(2021).
The Interaction of Porcine Dihydropyrimidine Dehydrogenase with the Chemotherapy Sensitizer: 5-Ethynyluracil.
Biochemistry,
60,
1120-1132.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
30-Jan-21
|
Release date:
|
07-Apr-21
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q28943
(DPYD_PIG) -
Dihydropyrimidine dehydrogenase [NADP(+)] from Sus scrofa
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
1025 a.a.
1005 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.3.1.2
- dihydropyrimidine dehydrogenase (NADP(+)).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
5,6-dihydrouracil + NADP+ = uracil + NADPH + H+
|
 |
 |
 |
 |
 |
5,6-dihydrouracil
Bound ligand (Het Group name = )
matches with 80.00% similarity
|
+
|
NADP(+)
Bound ligand (Het Group name = )
matches with 71.19% similarity
|
=
|
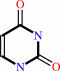 uracil
uracil
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
60:1120-1132
(2021)
|
|
PubMed id:
|
|
|
|

|
| |
|
The Interaction of Porcine Dihydropyrimidine Dehydrogenase with the Chemotherapy Sensitizer: 5-Ethynyluracil.
|
|
D.C.Forouzesh,
B.A.Beaupre,
A.Butrin,
Z.Wawrzak,
D.Liu,
G.R.Moran.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Dihydropyrimidine dehydrogenase (DPD) is a complex enzyme that reduces the
5,6-vinylic bond of pyrimidines, uracil, and thymine. 5-Fluorouracil (5FU) is
also a substrate for DPD and a common chemotherapeutic agent used to treat
numerous cancers. The reduction of 5FU to 5-fluoro-5,6-dihydrouracil negates its
toxicity and efficacy. Patients with high DPD activity levels typically have
poor outcomes when treated with 5FU. DPD is thus a central mitigating factor in
the treatment of a variety of cancers. 5-Ethynyluracil (5EU) covalently
inactivates DPD by cross-linking with the active-site general acid cysteine in
the pyrimidine binding site. This reaction is dependent on the simultaneous
binding of 5EU and nicotinamide adenine dinucleotide phosphate (NADPH). This
ternary complex induces DPD to become activated by taking up two electrons from
the NADPH. The covalent inactivation of DPD by 5EU occurs concomitantly with
this reductive activation with a rate constant of ∼0.2 s-1. This
kinact value is correlated with the rate of reduction of one
of the two flavin cofactors and the localization of a mobile loop in the
pyrimidine active site that places the cysteine that serves as the general acid
in catalysis proximal to the 5EU ethynyl group. Efficient cross-linking is
reliant on enzyme activation, but this process appears to also have a
conformational aspect in that nonreductive NADPH analogues can also induce a
partial inactivation. Cross-linking then renders DPD inactive by severing the
proton-coupled electron transfer mechanism that transmits electrons 56 Å across
the protein.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

