 |
PDBsum entry 7cpm
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of dodecaprenyl diphosphate synthase from thermobifida fusca
|
|
Structure:
|
 |
Trans,polycis-polyprenyl diphosphate synthase ((2z,6e)- farnesyl diphosphate specific). Chain: a, b, c, d, e, f. Synonym: cis-prenyltransferase,dodecaprenyl diphosphate synthase. Engineered: yes
|
|
Source:
|
 |
Thermobifida fusca (strain yx). Organism_taxid: 269800. Strain: yx. Gene: tfu_0853. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.60Å
|
R-factor:
|
0.189
|
R-free:
|
0.234
|
|
|
Authors:
|
 |
H.Kurokawa,T.Ambo,S.Takahashi,T.Koyama
|
|
Key ref:
|
 |
H.Kurokawa
et al.
(2020).
Crystal structure of Thermobifida fusca cis-prenyltransferase reveals the dynamic nature of its RXG motif-mediated inter-subunit interactions critical for its catalytic activity.
Biochem Biophys Res Commun,
532,
459-465.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
07-Aug-20
|
Release date:
|
14-Oct-20
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q47RM6
(DPDP_THEFY) -
Trans,polycis-polyprenyl diphosphate synthase ((2Z,6E)-farnesyl diphosphate specific) from Thermobifida fusca (strain YX)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
282 a.a.
244 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.5.1.88
- trans,polycis-polyprenyl diphosphate synthase [(2Z,6E)-farnesyl
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
(2Z,6E)-farnesyl diphosphate + 9 isopentenyl diphosphate = di-trans,nona-cis-dodecaprenyl diphosphate + 9 diphosphate
|
|
2.
|
(2Z,6E)-farnesyl diphosphate + 10 isopentenyl diphosphate = di-trans,deca-cis-tridecaprenyl diphosphate + 10 diphosphate
|
|
3.
|
(2Z,6E)-farnesyl diphosphate + 11 isopentenyl diphosphate = di-trans,undeca-cis-tetradecaprenyl diphosphate + 11 diphosphate
|
|
 |
 |
 |
 |
 |
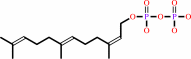 (2Z,6E)-farnesyl diphosphate
(2Z,6E)-farnesyl diphosphate
|
+
|
 9
×
isopentenyl diphosphate
9
×
isopentenyl diphosphate
|
=
|
di-trans,nona-cis-dodecaprenyl diphosphate
|
+
|
 9
×
diphosphate
9
×
diphosphate
|
|
 |
 |
 |
 |
 |
 (2Z,6E)-farnesyl diphosphate
(2Z,6E)-farnesyl diphosphate
|
+
|
 10
×
isopentenyl diphosphate
10
×
isopentenyl diphosphate
|
=
|
di-trans,deca-cis-tridecaprenyl diphosphate
|
+
|
 10
×
diphosphate
10
×
diphosphate
|
|
 |
 |
 |
 |
 |
 (2Z,6E)-farnesyl diphosphate
(2Z,6E)-farnesyl diphosphate
|
+
|
 11
×
isopentenyl diphosphate
11
×
isopentenyl diphosphate
|
=
|
di-trans,undeca-cis-tetradecaprenyl diphosphate
|
+
|
 11
×
diphosphate
11
×
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochem Biophys Res Commun
532:459-465
(2020)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of Thermobifida fusca cis-prenyltransferase reveals the dynamic nature of its RXG motif-mediated inter-subunit interactions critical for its catalytic activity.
|
|
H.Kurokawa,
T.Ambo,
S.Takahashi,
T.Koyama.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
cis-Prenyltransferases (cis-PTs) catalyze consecutive condensations of
isopentenyl diphosphate to an allylic diphosphate acceptor to produce a linear
polyprenyl diphosphate of designated length. Dimer formation is a prerequisite
for cis-PTs to catalyze all cis-prenyl condensation reactions. The
structure-function relationship of a conserved C-terminal RXG motif in cis-PTs
that forms inter-subunit interactions and has a role in catalytic activity has
attracted much attention. Here, we solved the crystal structure of a
medium-chain cis-PT from Thermobifida fusca that produces dodecaprenyl
diphosphate as a polyprenoid glycan carrier for cell wall synthesis. The
structure revealed a characteristic dimeric architecture of cis-PTs in which a
rigidified RXG motif of one monomer formed inter-subunit hydrogen bonds with the
catalytic site of the other monomer, while the RXG motif of the latter remained
flexible. Careful analyses suggested the existence of a possible long-range
negative cooperativity between the two catalytic sites on the two monomeric
subunits that allowed the binding of one subunit to stabilize the formation of
the enzyme-substrate ternary complex and facilitated the release of Mg-PPi and
subsequent intra-molecular translocation at the counter subunit so that the
condensation reaction could occur in consecutive cycles. The current structure
reveals the dynamic nature of the RXG motif and provides a rationale for
pursuing further investigations to elucidate the inter-subunit cooperativity of
cis-PTs.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

