 |
PDBsum entry 7bpy

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transcription
|
PDB id
|
|

|

|
7bpy
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transcription
|
 |
|
Title:
|
 |
X-ray structure of human pparalpha ligand binding domain-clofibric acid-src1 coactivator peptide co-crystals obtained by delipidation and co-crystallization
|
|
Structure:
|
 |
Peroxisome proliferator-activated receptor alpha. Chain: a, c. Engineered: yes. 15-meric peptide from nuclear receptor coactivator 1. Chain: b, d. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Organism_taxid: 9606. Expressed in: escherichia coli. Expression_system_taxid: 562. Synthetic: yes. Human. Organism_taxid: 9606
|
|
Resolution:
|
 |
|
2.09Å
|
R-factor:
|
0.194
|
R-free:
|
0.216
|
|
|
Authors:
|
 |
S.Kamata,R.Ishikawa,M.Akahane,T.Oyama,I.Ishii
|
|
Key ref:
|
 |
S.Kamata
et al.
(2020).
PPARα Ligand-Binding Domain Structures with Endogenous Fatty Acids and Fibrates.
iScience,
23,
101727.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
23-Mar-20
|
Release date:
|
11-Nov-20
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q07869
(PPARA_HUMAN) -
Peroxisome proliferator-activated receptor alpha from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
468 a.a.
271 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains B, D:
E.C.2.3.1.48
- histone acetyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-lysyl-[protein] + acetyl-CoA = N6-acetyl-L-lysyl-[protein] + CoA + H+
|
 |
 |
 |
 |
 |
L-lysyl-[protein]
|
+
|
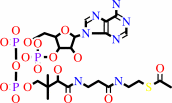 acetyl-CoA
acetyl-CoA
|
=
|
N(6)-acetyl-L-lysyl-[protein]
|
+
|
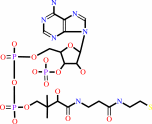 CoA
CoA
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
iScience
23:101727
(2020)
|
|
PubMed id:
|
|
|
|

|
| |
|
PPARα Ligand-Binding Domain Structures with Endogenous Fatty Acids and Fibrates.
|
|
S.Kamata,
T.Oyama,
K.Saito,
A.Honda,
Y.Yamamoto,
K.Suda,
R.Ishikawa,
T.Itoh,
Y.Watanabe,
T.Shibata,
K.Uchida,
M.Suematsu,
I.Ishii.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Most triacylglycerol-lowering fibrates have been developed in the 1960s-1980s
before their molecular target, peroxisome proliferator-activated receptor alpha
(PPARα), was identified. Twenty-one ligand-bound PPARα structures have been
deposited in the Protein Data Bank since 2001; however, binding modes of
fibrates and physiological ligands remain unknown. Here we show thirty-four
X-ray crystallographic structures of the PPARα ligand-binding domain, which are
composed of a "Center" and four "Arm" regions, in complexes
with five endogenous fatty acids, six fibrates in clinical use, and six
synthetic PPARα agonists. High-resolution structural analyses, in combination
with coactivator recruitment and thermostability assays, demonstrate that
stearic and palmitic acids are presumably physiological ligands; coordination to
Arm III is important for high PPARα potency/selectivity of pemafibrate and
GW7647; and agonistic activities of four fibrates are enhanced by the partial
agonist GW9662. These results renew our understanding of PPARα ligand
recognition and contribute to the molecular design of next-generation
PPAR-targeted drugs.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| |

