 |
PDBsum entry 7a3v
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.4.2.29
- tRNA-guanosine(34) preQ1 transglycosylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
7-aminomethyl-7-carbaguanine + guanosine34 in tRNA = 7-aminomethyl-7- carbaguanosine34 in tRNA + guanine
|
 |
 |
 |
 |
 |
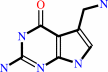 7-aminomethyl-7-carbaguanine
7-aminomethyl-7-carbaguanine
|
+
|
guanosine(34) in tRNA
|
=
|
7-aminomethyl-7- carbaguanosine(34) in tRNA
|
+
|
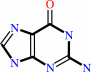 guanine
guanine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
ACS Chem Biol
16:1090-1098
(2021)
|
|
PubMed id:
|
|
|
|

|
| |
|
Targeting a Cryptic Pocket in a Protein-Protein Contact by Disulfide-Induced Rupture of a Homodimeric Interface.
|
|
D.Nguyen,
X.Xie,
S.Jakobi,
F.Terwesten,
A.Metz,
T.X.P.Nguyen,
V.A.Palchykov,
A.Heine,
K.Reuter,
G.Klebe.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Interference with protein-protein interfaces represents an attractive as well as
challenging option for therapeutic intervention and drug design. The enzyme
tRNA-guanine transglycosylase, a target to fight Shigellosis, is only functional
as a homodimer. Although we previously produced monomeric variants by
site-directed mutagenesis, we only crystallized the functional dimer, simply
because upon crystallization the local protein concentration increases and
favors formation of the dimer interface, which represents an optimal and highly
stable packing of the protein in the solid state. Unfortunately, this prevents
access to structural information about the interface geometry in its monomeric
state and complicates the development of modulators that can interfere with and
prevent dimer formation. Here, we report on a cysteine-containing protein
variant in which, under oxidizing conditions, a disulfide linkage is formed.
This reinforces a novel packing geometry of the enzyme. In this captured
quasi-monomeric state, the monomer units arrange in a completely different way
and, thus, expose a loop-helix motif, originally embedded into the old
interface, now to the surface. The motif adopts a geometry incompatible with the
original dimer formation. Via the soaking of fragments into the crystals, we
identified several hits accommodating a cryptic binding site next to the
loop-helix motif and modulated its structural features. Our study demonstrates
the druggability of the interface by breaking up the homodimeric protein using
an introduced disulfide cross-link. By rational concepts, we increased the
potency of these fragments to a level where we confirmed their binding by NMR to
a nondisulfide-linked TGT variant. The idea of intermediately introducing a
disulfide linkage may serve as a general concept of how to transform a homodimer
interface into a quasi-monomeric state and give access to essential structural
and design information.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

