 |
PDBsum entry 6vgo
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.4.13.19
- membrane dipeptidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an L-aminoacyl-L-amino acid + H2O = 2 an L-alpha-amino acid
|
 |
 |
 |
 |
 |
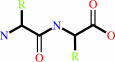 L-aminoacyl-L-amino acid
L-aminoacyl-L-amino acid
|
+
|
H2O
|
=
|
2
×
an L-alpha-amino acid
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Struct Biol
211:107512
(2020)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of human DPEP3 in complex with the SC-003 antibody Fab fragment reveals basis for lack of dipeptidase activity.
|
|
K.Hayashi,
K.L.Longenecker,
P.Koenig,
A.Prashar,
J.Hampl,
V.Stoll,
S.Vivona.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Dipeptidase 3 (DPEP3) is one of three glycosylphosphatidylinositol-anchored
metallopeptidases potentially involved in the hydrolytic metabolism of
dipeptides. While its exact biological function is not clear, DPEP3 expression
is normally limited to testis, but can be elevated in ovarian cancer. Antibody
drug conjugates targeting DPEP3 have shown efficacy in preclinical models with a
pyrrolobenzodiazepine conjugate, SC-003, dosed in a phase I clinical trial
(NCT02539719). Here we reveal the novel atomic structure of DPEP3 alone and in
complex with the SC-003 Fab fragment at 1.8 and 2.8 Å, respectively. The
structure of DPEP3/SC-003 Fab complex reveals an eighteen-residue epitope across
the DPEP3 dimerization interface distinct from the enzymatic active site. DPEP1
and DPEP3 extracellular domains share a conserved, dimeric TIM (β/α)8-barrel
fold, consistent with 49% sequence identity. However, DPEP3 diverges from DPEP1
and DPEP2 in key positions of its active site: a histidine to tyrosine variation
at position 269 reduces affinity for the β zinc and may cause substrate steric
hindrance, whereas an aspartate to asparagine change at position 359 abolishes
activation of the nucleophilic water/hydroxide, resulting in no in vitro
activity against a variety of dipeptides and biological substrates (imipenem,
leukotriene D4 and cystinyl-bis-glycine). Hence DPEP3, unlike DPEP1 and DPEP2,
may require an activating co-factor in vivo or may remain an inactive,
degenerate enzyme. This report sheds light on the structural discriminants
between active and inactive membrane dipeptidases and provides a benchmark to
characterize current and future DPEP3-targeted therapeutic approaches.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

