 |
PDBsum entry 6ihc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Biosynthetic protein
|
PDB id
|
|

|

|
6ihc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|





 (+ 0 more)
151 a.a.
(+ 0 more)
151 a.a.
|
 |

|

|

|

|

|

|

|
 65 a.a.
65 a.a.
|
 |

|

|
|
|
|
|
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Biosynthetic protein
|
 |
|
Title:
|
 |
Crystal structure of (3r)-hydroxyacyl-acyl carrier protein dehydratase(fabz) y100a mutant in complex with holo-acp from helicobacter pylori
|
|
Structure:
|
 |
3-hydroxyacyl-[acyl-carrier-protein] dehydratase fabz. Chain: a, b, c, d, e, f. Synonym: (3r)-hydroxymyristoyl-[acyl-carrier-protein] dehydratase, (3r)-hydroxymyristoyl-acp dehydrase,beta-hydroxyacyl-acp dehydratase. Engineered: yes. Holo-form acyl carrier protein (holo-acp). Chain: g. Engineered: yes
|
|
Source:
|
 |
Helicobacter pylori. Campylobacter pylori. Organism_taxid: 210. Gene: fabz, aod77_0202395. Expressed in: escherichia coli. Expression_system_taxid: 562. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.40Å
|
R-factor:
|
0.177
|
R-free:
|
0.214
|
|
|
Authors:
|
 |
S.Q.Shen,L.Zhang,L.Zhang
|
|
Key ref:
|
 |
S.Shen
et al.
(2019).
A back-door Phenylalanine coordinates the stepwise hexameric loading of acyl carrier protein by the fatty acid biosynthesis enzyme β-hydroxyacyl-acyl carrier protein dehydratase (FabZ).
Int J Biol Macromol,
128,
5.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
29-Sep-18
|
Release date:
|
10-Apr-19
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D, E, F:
E.C.4.2.1.59
- 3-hydroxyacyl-[acyl-carrier-protein] dehydratase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a (3R)-hydroxyacyl-[ACP] = a (2E)-enoyl-[ACP] + H2O
|
 |
 |
 |
 |
 |
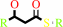 (3R)-3-hydroxyacyl-[acyl-carrier protein]
(3R)-3-hydroxyacyl-[acyl-carrier protein]
|
=
|
trans-2-enoyl-[acyl- carrier protein]
|
+
|
H(2)O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Int J Biol Macromol
128:5
(2019)
|
|
PubMed id:
|
|
|
|

|
| |
|
A back-door Phenylalanine coordinates the stepwise hexameric loading of acyl carrier protein by the fatty acid biosynthesis enzyme β-hydroxyacyl-acyl carrier protein dehydratase (FabZ).
|
|
S.Shen,
X.Hang,
J.Zhuang,
L.Zhang,
H.Bi,
L.Zhang.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The fatty acid biosynthesis pathway (FAS) was a fundamental procedure to
generate a diversity of lipid components for cellular metabolism in bacteria,
while the mechanism of substrate recognition remains unclear. The
β-hydroxyacyl-acyl carrier protein dehydratase hexamer (FabZ) is an essential
module in the elongation cycle of type-II FAS, catalyzing the dehydration of
β-hydroxyacyl-lipid substrate carried by the holo form acyl carrier protein
(holo-ACP). We previously elucidated an alternating seesaw-like ACP loading
manner within a FabZ dimer subunits, mediated by a front-door residue Tyrosine
(Tyr100). Here, we demonstrated that a back-door residue Phenylalanine (Phe83)
of FabZ regulates the stepwise hexameric loading of ACP. Our finding represents
clues as to the dynamic ACP recognition and catalysis mechanism of dehydratase
in fatty acid biosynthesis, and provides critical information for developing
antimicrobials targeting the dehydratase module in fatty acid biosynthesis
pathway.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

