 |
PDBsum entry 6i0c
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.8
- cholinesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an acylcholine + H2O = a carboxylate + choline + H+
|
 |
 |
 |
 |
 |
acylcholine
Bound ligand (Het Group name = )
matches with 46.67% similarity
|
+
|
H2O
|
=
|
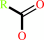 carboxylate
carboxylate
|
+
|
 choline
choline
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Eur J Med Chem
168:491-514
(2019)
|
|
PubMed id:
|
|
|
|

|
| |
|
Novel tacrine-tryptophan hybrids: Multi-target directed ligands as potential treatment for Alzheimer's disease.
|
|
K.Chalupova,
J.Korabecny,
M.Bartolini,
B.Monti,
D.Lamba,
R.Caliandro,
A.Pesaresi,
X.Brazzolotto,
A.J.Gastellier,
F.Nachon,
J.Pejchal,
M.Jarosova,
V.Hepnarova,
D.Jun,
M.Hrabinova,
R.Dolezal,
J.Zdarova Karasova,
M.Mzik,
Z.Kristofikova,
J.Misik,
L.Muckova,
P.Jost,
O.Soukup,
M.Benkova,
V.Setnicka,
L.Habartova,
M.Chvojkova,
L.Kleteckova,
K.Vales,
E.Mezeiova,
E.Uliassi,
M.Valis,
E.Nepovimova,
M.L.Bolognesi,
K.Kuca.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
A combination of tacrine and tryptophan led to the development of a new family
of heterodimers as multi-target agents with potential to treat Alzheimer's
disease. Based on the in vitro biological profile, compound S-K1035 was found
to be the most potent inhibitor of human acetylcholinesterase (hAChE) and human
butyrylcholinesterase (hBChE), demonstrating balanced IC50 values of
6.3 and 9.1 nM, respectively. For all the tacrine-tryptophan heterodimers,
favorable inhibitory effect on hAChE as well as on hBChE was coined to the
optimal spacer length ranging from five to eight carbon atoms between these two
pharmacophores. S-K1035 also showed good ability to inhibit Aβ42
self-aggregation (58.6 ± 5.1% at 50 μM) as well as hAChE-induced
Aβ40 aggregation (48.3 ± 6.3% at 100 μM). The X-ray
crystallographic analysis of TcAChE in complex with S-K1035 pinpointed the
utility of the hybridization strategy applied and the structures determined with
the two K1035 enantiomers in complex with hBChE could explain the higher
inhibition potency of S-K1035. Other in vitro evaluations predicted the ability
of S-K1035 to cross blood-brain barrier and to exert a moderate inhibition
potency against neuronal nitric oxide synthase. Based on the initial promising
biochemical data and a safer in vivo toxicity compared to tacrine, S-K1035 was
administered to scopolamine-treated rats being able to dose-dependently revert
amnesia.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

