 |
PDBsum entry 6fxh
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.7.1.77
- nucleoside phosphotransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 2'-deoxyribonucleoside + a ribonucleoside 5'-phosphate = a ribonucleoside + a 2'-deoxyribonucleoside 5'-phosphate
|
 |
 |
 |
 |
 |
2'-deoxyribonucleoside
|
+
|
ribonucleoside 5'-phosphate
|
=
|
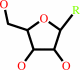 ribonucleoside
ribonucleoside
|
+
|
2'-deoxyribonucleoside 5'-phosphate
Bound ligand (Het Group name = )
matches with 60.00% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.3.1.3.5
- 5'-nucleotidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a ribonucleoside 5'-phosphate + H2O = a ribonucleoside + phosphate
|
 |
 |
 |
 |
 |
ribonucleoside 5'-phosphate
|
+
|
H2O
|
=
|
ribonucleoside
Bound ligand (Het Group name = )
matches with 60.00% similarity
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.3.1.3.99
- IMP-specific 5'-nucleotidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
IMP + H2O = inosine + phosphate
|
 |
 |
 |
 |
 |
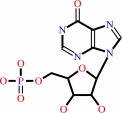 IMP
IMP
|
+
|
H2O
|
=
|
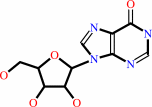 inosine
inosine
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Divalent metal cation
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Eur J Med Chem
168:28-44
(2019)
|
|
PubMed id:
|
|
|
|

|
| |
|
Lead optimization and biological evaluation of fragment-based cN-II inhibitors.
|
|
R.Guillon,
R.Rahimova,
Preeti,
D.Egron,
S.Rouanet,
C.Dumontet,
N.Aghajari,
L.P.Jordheim,
L.Chaloin,
S.Peyrottes.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The development of cytosolic 5'-nucleotidase II (cN-II) inhibitors is essential
to validate cN-II as a potential target for the reversion of resistance to
cytotoxic nucleoside analogues. We previously reported a fragment-based approach
combined with molecular modelling, herein, the selected hit-fragments were used
again in another computational approach based on the Ilib-diverse (a software
enabling to build virtual molecule libraries through fragment based de novo
design) program to generate a focused library of potential inhibitors. A
molecular scaffold related to a previously identified compound was selected and
led to a novel series of compounds. Ten out of nineteen derivatives showed
50-75% inhibition on the purified recombinant protein at 200 μM and among
them three derivatives (12, 13 and 18) exhibited Ki in the
sub-millimolar range (0.84, 2.4 and 0.58 mM, respectively). Despite their only
modest potency, the cN-II inhibitors showed synergistic effects when used in
combination with cytotoxic purine nucleoside analogues on cancer cells.
Therefore, these derivatives represent a family of non-nucleos(t)idic cN-II
inhibitors with potential usefulness to overcome cancer drug resistance
especially in hematological malignancies in which cN-II activity has been
described as an important parameter.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

