 |
PDBsum entry 6g4n
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Torpedo californica acetylcholinesterase bound to uncharged hybrid reactivator 2
|
|
Structure:
|
 |
Acetylcholinesterase. Chain: a, b. Synonym: ache. Ec: 3.1.1.7
|
|
Source:
|
 |
Tetronarce californica. Pacific electric ray. Organism_taxid: 7787
|
|
Resolution:
|
 |
|
2.90Å
|
R-factor:
|
0.189
|
R-free:
|
0.271
|
|
|
Authors:
|
 |
G.Santoni,E.De La Mora,J.De Souza,I.Silman,J.Sussman,R.Baati,M.Weik, F.Nachon
|
|
Key ref:
|
 |
G.Santoni
et al.
(2018).
Structure-Based Optimization of Nonquaternary Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents.
J Med Chem,
61,
7630-7639.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
28-Mar-18
|
Release date:
|
29-Aug-18
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P04058
(ACES_TETCF) -
Acetylcholinesterase from Tetronarce californica
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
586 a.a.
532 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.7
- acetylcholinesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
acetylcholine + H2O = choline + acetate + H+
|
 |
 |
 |
 |
 |
acetylcholine
Bound ligand (Het Group name = )
matches with 41.18% similarity
|
+
|
H2O
|
=
|
 choline
choline
|
+
|
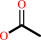 acetate
acetate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
61:7630-7639
(2018)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure-Based Optimization of Nonquaternary Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents.
|
|
G.Santoni,
J.de Sousa,
E.de la Mora,
J.Dias,
L.Jean,
J.L.Sussman,
I.Silman,
P.Y.Renard,
R.C.D.Brown,
M.Weik,
R.Baati,
F.Nachon.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Acetylcholinesterase (AChE), a key enzyme in the central and peripheral nervous
systems, is the principal target of organophosphorus nerve agents. Quaternary
oximes can regenerate AChE activity by displacing the phosphyl group of the
nerve agent from the active site, but they are poorly distributed in the central
nervous system. A promising reactivator based on tetrahydroacridine linked to a
nonquaternary oxime is also an undesired submicromolar reversible inhibitor of
AChE. X-ray structures and molecular docking indicate that structural
modification of the tetrahydroacridine might decrease inhibition without
affecting reactivation. The chlorinated derivative was synthesized and, in line
with the prediction, displayed a 10-fold decrease in inhibition but no
significant decrease in reactivation efficiency. X-ray structures with the
derivative rationalize this outcome. We thus show that rational design based on
structural studies permits the refinement of new-generation pyridine aldoxime
reactivators that may be more effective in the treatment of nerve agent
intoxication.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

