 |
PDBsum entry 5v3c
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.4.2.29
- tRNA-guanosine(34) preQ1 transglycosylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
7-aminomethyl-7-carbaguanine + guanosine34 in tRNA = 7-aminomethyl-7- carbaguanosine34 in tRNA + guanine
|
 |
 |
 |
 |
 |
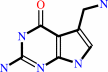 7-aminomethyl-7-carbaguanine
7-aminomethyl-7-carbaguanine
|
+
|
guanosine(34) in tRNA
|
=
|
7-aminomethyl-7- carbaguanosine(34) in tRNA
|
+
|
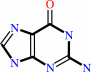 guanine
guanine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
ChemMedChem
15:324-337
(2020)
|
|
PubMed id:
|
|
|
|

|
| |
|
Fragments as Novel Starting Points for tRNA-Guanine Transglycosylase Inhibitors Found by Alternative Screening Strategies.
|
|
E.Hassaan,
P.O.Eriksson,
S.Geschwindner,
A.Heine,
G.Klebe.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Crystallography provides structural information crucial for fragment
optimization, however several criteria must be met to screen directly on protein
crystals as soakable, well-diffracting specimen must be available. We screened a
96-fragment library against the tRNA-modifying enzyme TGT using crystallography.
Eight hits, some with surprising binding poses, were detected. However, the
amount of data collection, reduction and refinement is assumed substantial.
Therefore, having a reliable cascade of fast and cost-efficient methods
available for pre-screening before embarking to elaborate crystallographic
screening appears beneficial. This allows filtering of compounds to the most
promising hits, available to rapidly progress from hit-to-lead. But how to
ensure that this workflow is reliable? To answer this question, we also applied
SPR and NMR to the same screening sample to study whether identical hits are
retrieved. Upon hit-list comparisons, crystallography shows with NMR and SPR,
only one overlapping hit and all three methods shared no common hits. This
questions a cascade-type screening protocol at least in the current example.
Compared to crystallography, SPR and NMR detected higher percentages of
non-active-site binders suggesting the importance of running reporter
ligand-based competitive screens in SPR and NMR, a requirement not needed in
crystallography. Although not specific, NMR proved a more sensitive method
relative to SPR and crystallography, as it picked up the highest numbers of
binders.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

