 |
PDBsum entry 5trc
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Crystal structure of phosphorylated ac3-ac5 domains of yeast acetyl- coa carboxylase
|
|
Structure:
|
 |
Acetyl-coa carboxylase. Chain: a, b. Synonym: acc,fatty acid synthetase 3,mRNA transport-defective protein 7. Engineered: yes
|
|
Source:
|
 |
Saccharomyces cerevisiae (strain atcc 204508 / s288c). Baker's yeast. Organism_taxid: 559292. Strain: atcc 204508 / s288c. Gene: acc1, abp2, fas3, mtr7, ynr016c, n3175. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
2.90Å
|
R-factor:
|
0.226
|
R-free:
|
0.287
|
|
|
Authors:
|
 |
J.Wei,L.Tong
|
|
Key ref:
|
 |
J.Wei
et al.
(2016).
A unified molecular mechanism for the regulation of acetyl-CoA carboxylase by phosphorylation.
Cell Discov,
2,
16044.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
26-Oct-16
|
Release date:
|
07-Dec-16
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B:
E.C.6.3.4.14
- biotin carboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N6-biotinyl-L-lysyl-[protein] + hydrogencarbonate + ATP = N6- carboxybiotinyl-L-lysyl-[protein] + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
N(6)-biotinyl-L-lysyl-[protein]
|
+
|
 hydrogencarbonate
hydrogencarbonate
|
+
|
 ATP
ATP
|
=
|
N(6)- carboxybiotinyl-L-lysyl-[protein]
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains A, B:
E.C.6.4.1.2
- acetyl-CoA carboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
hydrogencarbonate + acetyl-CoA + ATP = malonyl-CoA + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 hydrogencarbonate
hydrogencarbonate
|
+
|
 acetyl-CoA
acetyl-CoA
|
+
|
 ATP
ATP
|
=
|
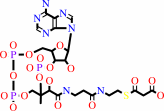 malonyl-CoA
malonyl-CoA
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Biotin
|
 |
 |
 |
 |
 |
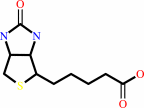 Biotin
Biotin
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Cell Discov
2:16044
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
A unified molecular mechanism for the regulation of acetyl-CoA carboxylase by phosphorylation.
|
|
J.Wei,
Y.Zhang,
T.Y.Yu,
K.Sadre-Bazzaz,
M.J.Rudolph,
G.A.Amodeo,
L.S.Symington,
T.Walz,
L.Tong.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

