 |
PDBsum entry 5std

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Lyase
|
 |
|
Title:
|
 |
Scytalone dehydratase plus inhibitor 2
|
|
Structure:
|
 |
Scytalone dehydratase. Chain: a, b, c. Synonym: sdh. Engineered: yes
|
|
Source:
|
 |
Magnaporthe grisea. Organism_taxid: 148305. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Biol. unit:
|
 |
 Hexamer (from
)
Hexamer (from
)
|
|
Resolution:
|
 |
|
1.95Å
|
R-factor:
|
0.212
|
R-free:
|
0.259
|
|
|
Authors:
|
 |
Z.Wawrzak,T.Sandalova,J.J.Steffens,G.S.Basarab,T.Lundqvist, Y.Lindqvist,D.B.Jordan
|
Key ref:

|
 |
Z.Wawrzak
et al.
(1999).
High-resolution structures of scytalone dehydratase-inhibitor complexes crystallized at physiological pH.
Proteins,
35,
425-439.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
10-Feb-99
|
Release date:
|
29-Dec-99
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P56221
(SCYD_MAGO7) -
Scytalone dehydratase from Magnaporthe oryzae (strain 70-15 / ATCC MYA-4617 / FGSC 8958)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
172 a.a.
164 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.2.1.94
- scytalone dehydratase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
scytalone = 1,3,8-trihydroxynaphthalene + H2O
|
 |
 |
 |
 |
 |
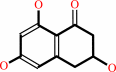 scytalone
scytalone
|
=
|
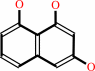 1,3,8-trihydroxynaphthalene
1,3,8-trihydroxynaphthalene
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proteins
35:425-439
(1999)
|
|
PubMed id:
|
|
|
|

|
| |
|
High-resolution structures of scytalone dehydratase-inhibitor complexes crystallized at physiological pH.
|
|
Z.Wawrzak,
T.Sandalova,
J.J.Steffens,
G.S.Basarab,
T.Lundqvist,
Y.Lindqvist,
D.B.Jordan.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Scytalone dehydratase is a molecular target of inhibitor design efforts aimed at
preventing the fungal disease caused by Magnaporthe grisea. A method for
cocrystallization of enzyme with inhibitors at neutral pH has produced several
crystal structures of enzyme-inhibitor complexes at resolutions ranging from 1.5
to 2.2 A. Four high resolution structures of different enzyme-inhibitor
complexes are described. In contrast to the original X-ray structure of the
enzyme, the four new structures have well-defined electron density for the loop
region comprising residues 115-119 and a different conformation between residues
154 and 160. The structure of the enzyme complex with an aminoquinazoline
inhibitor showed that the inhibitor is in a position to form a hydrogen bond
with the amide of the Asn131 side chain and with two water molecules in a
fashion similar to the salicylamide inhibitor in the original structure, thus
confirming design principles. The aminoquinazoline structure also allows for a
more confident assignment of donors and acceptors in the hydrogen bonding
network. The structures of the enzyme complexes with two dichlorocyclopropane
carboxamide inhibitors showed the two chlorine atoms nearly in plane with the
amide side chain of Asn131. The positions of Phe53 and Phe158 are significantly
altered in the new structures in comparison to the two structures obtained from
crystals grown at acidic pH. The multiple structures help define the mobility of
active site amino acids critical for catalysis and inhibitor binding.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. The fungal melanin biosynthetic pathway. 4HNR,
tetrahydroxynapthalene reductase; SD, scytalone dehydratase;
3HNR, trihydroxynapthalene reductase.
|
 |
Figure 3.
Figure 3. Stereo views of the electron densities (2F[O] - F[C])
surrounding the inhibitors and the two water molecules in the
active site of scytalone dehydratase. (A) Inhibitor 1. (B)
Inhibitor 2. (C) Inhibitor 3. (D) Inhibitor 4.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from John Wiley & Sons, Inc.:
Proteins
(1999,
35,
425-439)
copyright 1999.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.J.Cottrell,
V.J.Gillet,
R.Taylor,
and
D.J.Wilton
(2004).
Generation of multiple pharmacophore hypotheses using multiobjective optimisation techniques.
|
| |
J Comput Aided Mol Des,
18,
665-682.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

