 |
PDBsum entry 5oy2

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
5oy2
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.11.2.1
- unspecific peroxygenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RH + H2O2 = ROH + H2O
|
 |
 |
 |
 |
 |
RH
|
+
|
 H2O2
H2O2
|
=
|
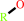 ROH
ROH
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Heme-thiolate
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
ACS Chem Biol
13:3259-3268
(2018)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural Insights into the Substrate Promiscuity of a Laboratory-Evolved Peroxygenase.
|
|
M.Ramirez-Escudero,
P.Molina-Espeja,
P.Gomez de Santos,
M.Hofrichter,
J.Sanz-Aparicio,
M.Alcalde.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Because of their minimal requirements, substrate promiscuity and product
selectivity, fungal peroxygenases are now considered to be the jewel in the
crown of C-H oxyfunctionalization biocatalysts. In this work, the crystal
structure of the first laboratory-evolved peroxygenase expressed by yeast was
determined at a resolution of 1.5 Å. Notable differences were detected between
the evolved and native peroxygenase from Agrocybe aegerita, including the
presence of a full N-terminus and a broader heme access channel due to the
mutations that accumulated through directed evolution. Further mutagenesis and
soaking experiments with a palette of peroxygenative and peroxidative substrates
suggested dynamic trafficking through the heme channel as the main driving force
for the exceptional substrate promiscuity of peroxygenase. Accordingly, this
study provides the first structural evidence at an atomic level regarding the
mode of substrate binding for this versatile biocatalyst, which is discussed
within a biological and chemical context.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

