 |
PDBsum entry 5oit

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
5oit
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Inha (t2a mutant) complexed with 5-((5-amino-3-methyl-1h-pyrazol-1- yl)methyl)-n-(1-(2-chloro-6-fluorobenzyl)-1h-pyrazol-3-yl)-1,3,4- thiadiazol-2-amine
|
|
Structure:
|
 |
Enoyl-[acyl-carrier-protein] reductase [nadh]. Chain: b, d, f, h. Synonym: enoyl-acp reductase,fas-ii enoyl-acp reductase,nadh- dependent 2-trans-enoyl-acp reductase. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Mycobacterium tuberculosis. Organism_taxid: 83332. Gene: inha, rv1484, mtcy277.05. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.58Å
|
R-factor:
|
0.170
|
R-free:
|
0.199
|
|
|
Authors:
|
 |
M.A.Convery
|
|
Key ref:
|
 |
F.Prati
et al.
(2018).
Screening of a Novel Fragment Library with Functional Complexity against Mycobacterium tuberculosis InhA.
ChemMedChem,
13,
672-677.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
19-Jul-17
|
Release date:
|
14-Feb-18
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P9WGR1
(INHA_MYCTU) -
Enoyl-[acyl-carrier-protein] reductase [NADH] from Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
269 a.a.
268 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.3.1.9
- enoyl-[acyl-carrier-protein] reductase (NADH).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 2,3-saturated acyl-[ACP] + NAD+ = a (2E)-enoyl-[ACP] + NADH + H+
|
 |
 |
 |
 |
 |
2,3-saturated acyl-[ACP]
|
+
|
NAD(+)
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
(2E)-enoyl-[ACP]
|
+
|
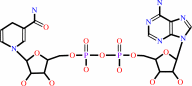 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
ChemMedChem
13:672-677
(2018)
|
|
PubMed id:
|
|
|
|

|
| |
|
Screening of a Novel Fragment Library with Functional Complexity against Mycobacterium tuberculosis InhA.
|
|
F.Prati,
F.Zuccotto,
D.Fletcher,
M.A.Convery,
R.Fernandez-Menendez,
R.Bates,
L.Encinas,
J.Zeng,
C.W.Chung,
P.De Dios Anton,
A.Mendoza-Losana,
C.Mackenzie,
S.R.Green,
M.Huggett,
D.Barros,
P.G.Wyatt,
P.C.Ray.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Our findings reported herein provide support for the benefits of including
functional group complexity (FGC) within fragments when screening against
protein targets such as Mycobacterium tuberculosis InhA. We show that InhA
fragment actives with FGC maintained their binding pose during elaboration.
Furthermore, weak fragment hits with functional group handles also allowed for
facile fragment elaboration to afford novel and potent InhA inhibitors with good
ligand efficiency metrics for optimization.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

