 |
PDBsum entry 5in8

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Lyase/lyase inhibitor
|
PDB id
|
|

|

|
5in8
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.2.3.9
- aristolochene synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Germacrene derived sesquiterpenoid biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(2E,6E)-farnesyl diphosphate = +-aristolochene + diphosphate
|
 |
 |
 |
 |
 |
 (2E,6E)-farnesyl diphosphate
(2E,6E)-farnesyl diphosphate
|
=
|
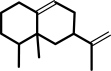 (+)-aristolochene
(+)-aristolochene
|
+
|
diphosphate
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mg(2+); Mn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
55:2864-2874
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Probing the Role of Active Site Water in the Sesquiterpene Cyclization Reaction Catalyzed by Aristolochene Synthase.
|
|
M.Chen,
W.K.Chou,
N.Al-Lami,
J.A.Faraldos,
R.K.Allemann,
D.E.Cane,
D.W.Christianson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Aristolochene synthase (ATAS) is a high-fidelity terpenoid cyclase that converts
farnesyl diphosphate exclusively into the bicyclic hydrocarbon aristolochene.
Previously determined crystal structures of ATAS complexes revealed trapped
active site water molecules that could potentially interact with catalytic
intermediates: water "w" hydrogen bonds with S303 and N299, water
molecules "w1" and "w2" hydrogen bond with Q151, and a
fourth water molecule coordinates to the Mg(2+)C ion. There is no obvious role
for water in the ATAS mechanism because the enzyme exclusively generates a
hydrocarbon product. Thus, these water molecules are tightly controlled so that
they cannot react with carbocation intermediates. Steady-state kinetics and
product distribution analyses of eight ATAS mutants designed to perturb
interactions with active site water molecules (S303A, S303H, S303D, N299A,
N299L, N299A/S303A, Q151H, and Q151E) indicate relatively modest effects on
catalysis but significant effects on sesquiterpene product distributions. X-ray
crystal structures of S303A, N299A, N299A/S303A, and Q151H mutants reveal
minimal perturbation of active site solvent structure. Seven of the eight
mutants generate farnesol and nerolidol, possibly resulting from addition of the
Mg(2+)C-bound water molecule to the initially formed farnesyl cation, but no
products are generated that would suggest enhanced reactivity of other active
site water molecules. However, intermediate germacrene A tends to accumulate in
these mutants. Thus, apart from the possible reactivity of Mg(2+)C-bound water,
active site water molecules in ATAS are not directly involved in the chemistry
of catalysis but instead contribute to the template that governs the
conformation of the flexible substrate and carbocation intermediates.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

