 |
PDBsum entry 5df7
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of penicillin-binding protein 3 from pseudomonas aeruginosa in complex with azlocillin
|
|
Structure:
|
 |
Cell division protein. Chain: a, b. Synonym: penicillin-binding protein 3,pseudomonas aeruginosa genome assembly pae221. Engineered: yes
|
|
Source:
|
 |
Pseudomonas aeruginosa. Organism_taxid: 287. Gene: pbpb, ftsi_2, ers445055_04698, pae221_03076, yq19_27590. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008
|
|
Resolution:
|
 |
|
2.00Å
|
R-factor:
|
0.199
|
R-free:
|
0.237
|
|
|
Authors:
|
 |
J.Ren,J.E.Nettleship,A.Males,D.I.Stuart,R.J.Owens
|
|
Key ref:
|
 |
J.Ren
et al.
(2016).
Crystal structures of penicillin-binding protein 3 in complexes with azlocillin and cefoperazone in both acylated and deacylated forms.
Febs Lett,
590,
288-297.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
26-Aug-15
|
Release date:
|
13-Jan-16
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.3.4.16.4
- serine-type D-Ala-D-Ala carboxypeptidase.
|
|
 |
 |
 |
 |
 |
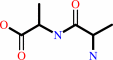
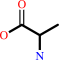
Reaction:
|
 |
D-alanyl-D-alanine + H2O = 2 D-alanine
|
 |
 |
 |
 |
 |
|
+
|
|
=
|
 2
×
2
×
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Febs Lett
590:288-297
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of penicillin-binding protein 3 in complexes with azlocillin and cefoperazone in both acylated and deacylated forms.
|
|
J.Ren,
J.E.Nettleship,
A.Males,
D.I.Stuart,
R.J.Owens.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Penicillin-binding protein 3 (PBP3) from Pseudomonas aeruginosa is the molecular
target of β-lactam-based antibiotics. Structures of PBP3 in complexes with
azlocillin and cefoperazone, which are in clinical use for the treatment of
pseudomonad infections, have been determined to 2.0 Å resolution. Together with
data from other complexes, these structures identify a common set of residues
involved in the binding of β-lactams to PBP3. Comparison of wild-type and an
active site mutant (S294A) showed that increased thermal stability of PBP3
following azlocillin binding was entirely due to covalent binding to S294,
whereas cefoperazone binding produces some increase in stability without the
covalent link. Consistent with this, a third crystal structure was determined in
which the hydrolysis product of cefoperazone was noncovalently bound in the
active site of PBP3. This is the first structure of a complex between a
penicillin-binding protein and cephalosporic acid and may be important in the
design of new noncovalent PBP3 inhibitors.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

