 |
PDBsum entry 5b5t

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/hydrolase inhibitor
|
PDB id
|
|

|

|
5b5t
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B, C, D:
E.C.2.3.2.2
- gamma-glutamyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an N-terminal (5-L-glutamyl)-[peptide] + an alpha-amino acid = 5-L- glutamyl amino acid + an N-terminal L-alpha-aminoacyl-[peptide]
|
 |
 |
 |
 |
 |
N-terminal (5-L-glutamyl)-[peptide]
|
+
|
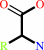 alpha-amino acid
alpha-amino acid
|
=
|
5-L- glutamyl amino acid
Bound ligand (Het Group name = )
matches with 40.00% similarity
|
+
|
N-terminal L-alpha-aminoacyl-[peptide]
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains A, B, C, D:
E.C.3.4.19.13
- glutathione gamma-glutamate hydrolase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
glutathione + H2O = L-cysteinylglycine + L-glutamate
|
|
2.
|
an S-substituted glutathione + H2O = an S-substituted L-cysteinylglycine + L-glutamate
|
|
 |
 |
 |
 |
 |
 glutathione
glutathione
|
+
|
H2O
|
=
|
L-cysteinylglycine
Bound ligand (Het Group name = )
matches with 40.91% similarity
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
S-substituted glutathione
|
+
|
H2O
|
=
|
S-substituted L-cysteinylglycine
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Bioorg Med Chem Lett
24:5340-5352
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Phosphonate-based irreversible inhibitors of human γ-glutamyl transpeptidase (GGT). GGsTop is a non-toxic and highly selective inhibitor with critical electrostatic interaction with an active-site residue Lys562 for enhanced inhibitory activity.
|
|
A.Kamiyama,
M.Nakajima,
L.Han,
K.Wada,
M.Mizutani,
Y.Tabuchi,
A.Kojima-Yuasa,
I.Matsui-Yuasa,
H.Suzuki,
K.Fukuyama,
B.Watanabe,
J.Hiratake.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

